Article from Townsend Letter
The clinical importance of 5alpha-reductase in human health and pathology,
part 2: Women – Polycystic Ovarian Syndrome, Premenstural Dysphoric Disorder, hormone replacement therapy and beyond.
by Alan B. McDaniel, MD
Introduction
Men and women have the same sex hormones, made from cholesterol by our ovaries or testicles and adrenal glands, also the brain… and even the skin! The body assumes male and female forms depending upon on how much of which hormone we make. This net effect is determined by enzymes, which produce our hormones and then alter them, to subtly “fine-tune” the messages they deliver. One such critical enzyme is 5alpha-reductase (5α-R): When its effects go awry, the consequences can be dramatic – altering the body viaits sex hormones and nervous system function through its neurosteroids.
This report describes women whose sex hormones are inappropriately 5α-reduced. The actions and effects of the involved hormones are discussed, as are the causes of the problems and various solutions. The new frontier of 5alpha-reduced neurosteroids is introduced. We examine the causal link between 5α-R and the significant health issues of premenstrual dysphoric disorder (PMDD) and premenstrual syndrome (PMS); painful menstruation; postpartum depression; “progesterone resistance” in hormone replacement therapy and even migraine headaches and seizures. Treatment options are presented and illustrated by these cases with references to published literature.
Women’s hormone replacement: Case 1
Case 1 is a petite (5’ 1”, 96 lbs.) woman who at age 41, in 2002 first saw Doc for thyroid trouble. Her history suggested she was insulin-resistant: In her teens, she had both menstrual irregularities requiring suppression with oral contraceptives (OCP) [i] and fibrocystic breast disease.[ii] These problems worsened in her late 30s, when after three children and a tubal ligation she developed menorrhagia with large clots, severe cramps and “alarming” mood swings. Her symptoms were mitigated by resuming the OCP but she never felt safe in using it.
Her hair was thinning excessively in ’02 – initially attributed to her thyroid problem. Symptoms of hypoglycemia sometimes interrupted her activities and forced her to snack. Her first degree-relatives’ history of obesity; hyperlipidemia; stroke; ovarian cysts and uterine fibroids also suggested inherited insulin resistance. These and symptoms implying the controversial diagnosis of “adrenal fatigue” indicated a workup beyond thyroid tests.
A 24 hour-urine adrenal steroid profile by GC-Mass Spec. showed robust cortisol and cortisone but low precursors and significantly, excessive 5α-R activity by increased androsterone relative to etiocholanolone. On 4 hour-oral glucose tolerance test with insulin, her fasting values of glucose (90 mg/dL) and insulin (3.2μU) were normal but her 30-min. insulin response was sluggish; the two hour glucose was excessive at 151 and she “felt like passing out” at the 4th hour, with glucose only 59 – a weak counter-regulatory response.[iii] Alas! The lab missed the “key” 2-hour insulin test, which if elevated might help explain this hypoglycemia.
Case 1 responded well to thyroid treatment combining T3 with T4; nutritional supplements and a healthy diet for insulin resistance (low glycemic index; slowly-accessible glucose and low insulinemic index). Her mental clarity was restored, her hair became normally thick and PMS and dysmenorrhea symptoms eased – until 2007, at age 46, when her Gynecologist (GYN) said she must stop the OCP.
Without the OCP, menorrhagia relapsed –heavy to “flooding.” Serious mood swings resumed, described as “outbursts.” Menstrual-related problems caused her to miss work every month. Her GYN prescribed conjugated equine estrogens and medroxyprogesterone (PremPro®). This failed: Case 1 felt worse and gained 10 lbs. (a lot when you weigh only 96!). Next, a surgical approach was recommended: The patient underwent endometrial ablation in 2008. She felt better postoperatively.
However, she did not feel well. In 2009, despite tests showing her ovarian cycles continued and produced normal and balanced amounts of steroid sex hormones, oral progesterone 100 mg daily was prescribed. This too was unsuccessful: It worsened symptoms and caused weight gain and so was discontinued. The GYN next recommended changing thyroid treatment to T4-only, which certainly failed to improve the situation.
Case 1 again consulted Doc in 2016. Now age 54, in addition to symptoms of thyroid dysfunction, she had also those of menopause: Vulvar atrophy, reduced libido, insomnia and cognitive difficulty. This was in spite of taking HRT from her GYN: Oral progesterone (Prometrium®) 100 mg at bedtime; transdermal estradiol (Evamist®) one spray and DHEA 10 mg every AM – and oral testosterone 3 mg every other morning. She added this clue: Her tests “always” showed low testosterone – but any more than her current dose caused her “serious acne” and undesirable hair growth!
Her blood was tested: Thyroid tests reaffirmed her dysfunctional deiodination – for which replacing some T4 with T3 compensated. Her sex hormones were unbalanced: Estradiol was 31.4 pg/mL, an “early follicular” value for cycling women. However, progesterone at 4.5 ng/mL is normal only in luteal phase. Her observation about low testosterone was almost true: Testosterone was immeasurably low (total <3 ng/dL and free <0.2 pg/mL) but dihydrotestosterone (DHT) was 3.5 ng/dL. Women usually have about half as much DHT as total testosterone; Case 1’s DHT was the greater – a decidedly non-physiological balance – showing abnormally induced 5α-R.
Hormones 101: Testosterone, DHT and estradiol
The differences between the sexes are determined in-utero and subsequently at adolescence through various factors, amongst which the enzyme 5α-reductase (5α-R) is important. Testosterone is not, as some believe, the Avatar of All Things Masculine. It is a pre-hormone, as noted in the companion to this paper.[iv]
Although the essence of testosterone is androgenic (literally: “makes men”), it quickly can be converted to the most powerfully feminizing hormone – estradiol. While men change a small but important fraction of their testosterone to estradiol,[v] women perform this step “wholesale,” giving them (at ovulation) up to ten times-more estradiol than healthy men ever have.
Conversely, men generously convert testosterone to DHT– while women should not. This difference is remarkably important, since DHT stimulates the Androgen Receptor (AR) in the cell nucleus approximately ten times-more potently than does testosterone.[vi] [vii] The AR, in turn, promotes the activation of all DNA programs that create the “masculine.” Normal men’s DHT can vary from 30-85 ng/dL, while women’s is considerably lower, between 4-22 ng/dL.[viii] In women, excessive DHT can produce such undesirable results as acne, unwanted facial and body hair and male-pattern hair loss – as Case 1 experienced.
Enzymes 101: 5α-Reductase (5α-R) and women
Enzymes are responsible for these normal – and abnormal – hormone effects. Enzymes are expediters of biochemical reactions. Generally proteins, they hasten the conversion of a raw material (or “substrate”) into something else, a “product.” The DNA genome encodes “blueprints” for enzymes. By transcribing, synthesizing, activating and degrading these enzymes – many of which have opposing or synergistic effects – our body makes products that manifest its structure, function and indeed, the continued existence of itself and its species.
Enzymes are “specialists.” The enzyme aromatase (CYP19A1, or estrogen synthase) converts testosterone to estradiol. As implied above, women normally make a lot of this enzyme and men not so much. The “title enzyme,” 5α-reductase to the contrary reduces testosterone to DHT – this enzyme is abundant in men, while healthy women produce little of it.
5α-Reductase exists to cut a double-bond between the carbon atoms #4 and #5 in the “A” ring of its substrates. These targets of 5α-R are formally called “3-keto, Δ 4, 5 steroids” (Figure 1). As above, one substrate is the hormone testosterone. Other hormones include aldosterone, cortisol and – drum-roll Maestro– progesterone. More substrates include hormone precursors (androstenedione, deoxycorticosterone) and “metabolites” (epi-testosterone).6
Figure 1: 5alpha-Reductase acts on 3-keto, Δ 4, 5 steroids
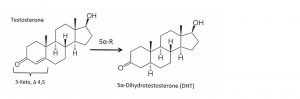
A more detailed review of 5α-R is presented in “part 1,” 4 and this is the clinical essence: There are three isoforms of 5α-reductase (different genomic encoding; dissimilar structure but nearly identical function)[ix] [x]and two other very different proteins also have similar effects. These five are so important they are found in allEukaryotic cells.6 The most recently discovered “type-3” isoform also seems to have an important role in glycosylation and genetically deficient-humans are severely impaired.[xi]
Isoforms of 5α-reductase are expressed in various tissues beginning rather shortly after conception and their appearance is orchestrated for quite specific moments in development. The products of 5α-R help direct the orderly differentiation of the brain and body into healthy men and women (psychosexual differentiation).[xii] For example, men lacking the genetic program for type-2 5α-R have pseudo-hermaphroditism, with ambiguous external genitalia and absent prostate gland. Women with the same defect, though, show but little evidence: Reduced body hair; freedom from acne – and on blood tests, high total testosterone/ DHT ratio.[xiii]
Enzyme production and function can be inappropriately increased. This paper focuses upon women in whom 5α-R is detrimentally over-produced. The author regrets that the reader must endure the following didactics to appreciate the consequences of this unfortunate situation – and to rejoice in the fact that blocking or inhibiting abnormal enzyme actions can give great relief.
Laboratory tests for 5α-reductase function
Practitioners cannot order an assay for 5α-R. Instead, commercially-available laboratory measures can indicate whether a person’s 5α-R activity is appropriate for their sex. Blood tests are used to find the absolute amounts and calculate the ratio of total – free and bound – testosterone (tTest) to total DHT. From normal reference intervals and clinical observation, it may be argued that women’s normal tTest/DHT value is about “2.”
A second laboratory metric is the balance of 5alpha- to 5beta-reduced steroid metabolites (e.g. androsterone to etiocholanolone) in GC/mass-spec studies of 24 hour-urine specimens. Men are expected to have more 5alpha-reduced products and women to show more 5beta. This method has been employed in published research[xiv] [xv] and these tests are available from several American reference laboratories – at least some of which have for years routinely report a calculated 5α/ 5β balance.[xvi] [xvii]
Case 1 revisited
Tests showed Case 1 excessively converts her testosterone to DHT (<3 ng/dL to 3.5 ng/dL; ratio tTest/DHT < 0.86). This explains her acne and crops of new hair growth with attempted testosterone replacement. Although a “backdoor pathway” to DHT has been demonstrated,[xviii] [xix] her unduly great amount is more likely the result of conversion by increased 5alpha-reduction.
The evidence? Excessive 5α-R activity was shown years before in her 24 hour-urine adrenal steroids: She had relatively high 5α-androsterone compared to the 5beta-reduced etiocholanolone. Her insulin resistance provides a causal mechanism, as follows. Moreover, her difficulties with progesterone also can be attributed to 5α-R. It is time to broaden our focus.
Enzymes 201: 5alpha-Reductase (5α-R) beyond testosterone
5α-Reductase acts on substrates other than testosterone: Aldosterone, like testosterone, may be more potent at its (single) receptor when 5alpha-reduced.6 [xx] Cortisol, in contrast, is weakened at its sole receptor – with important physiological effects.[xxi] The benefits of “diversifying signaling at a single nuclear receptor” can be invoked in all of these actions.[xxii] Progesterone, though, is neither intensified nor weakened by 5α-R but changed into something completely different, a neurosteroid.
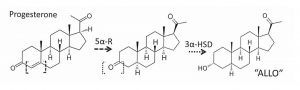
The first – and rate-limiting step – by which progesterone becomes a neurosteroid is performed by 5α-R. The second is via the unstinting cooperation of another enzyme, 3α-hydroxysteroid dehydrogenase (3α-HSD). This neurosteroid product has the unreasonably long proper name, 3α,5α-tetrahydro-progesterone (allopregnanolone), shortened hereafter to simply “ALLO” (Figure 2). The same two enzymes send an aldosterone precursor (deoxycorticosterone) to a similar neurosteroid, mercifully abbreviated THDOC.
Figure 2: 5α-Reductase and 3α-HSD convert progesterone to ALLO
Hormones 201: Neurosteroids
Neurosteroids are fairly recent arrivals upon the stage of physiological cognizance and merit careful attention. These are not the steroid hormones made in our body, which themselves will easily cross the blood-brain barrier to be “neuro-active” in the brain.
Neuro-active steroids such as testosterone and estrogens vary by sex, of course, and parents of adolescents know they are behavior-altering. They are also importantly neuro-protective.[xxiii] [xxiv] This helps explain the sex-differences in the onset, symptoms and outcomes of various neurological diseases such as Alzheimer’s; Parkinson’s; Huntington’s; multiple sclerosis; stroke; traumatic injury to brain and spinal cord; diabetic encephalopathy; peripheral neuropathy; seizures and psychiatric disorders.
Yet the brain doesn’t simply receive second-hand steroid hormones. Like the skin, it too has all the synthetic enzymes to make steroids de-novo from cholesterol – and this production is regulated by local factors, not the hypothalamic-pituitary axis.[xxv] [xxvi] “Neurosteroid” is the term applied to the neutrally-active steroids madewithin the brain. Importantly, the amount of ALLO within the brain depends upon the availability of progesterone and the activity of 5α-R – the “rate-limiting” enzyme.26
Brain cells containing this important pair of enzymes (5α-R, 3α-HSD) are found in regions critical for mood, emotion and sexual function. Their neurosteroid products, ALLO and THDOC act on the brain’s GABAA-receptor, once known to medical students as “the diazepam receptor.” Indeed, the neurotransmitter GABA, 5α-reduced neurosteroids, benzodiazepines, barbiturates and ethanol all exert similar and synergistic“mellowing” effects there – and more.[xxvii]
Hormones 301: Neurosteroids balance the brain– or not.
At the GABAA-receptor, ALLO and THDOC potently increase the inhibitory effects of GABA in its yin/ yang balance with glutamatergic stimulation.26 This balance is necessary for maintaining a network that successfully integrates stimuli with appropriate forebrain, limbic and HPA-axis responses. This also influences metabolic balance: The glucose counter-regulatory response is inhibited by hypothalamic GABAA-R; increasing this receptor’s output inhibits glucagon release with resulting hypoglycemia.[xxviii] [xxix] The modulation of GABAA-receptor function by neurosteroids is important.25
In acute stress, the proper amounts of ALLO and THDOC protectively reduce anxiety and stress-behavior.[xxx][xxxi] [xxxii] In chronic stress, inappropriate neurosteroids are associated with dysfunctional GABAergic transmission and increased susceptibility to stress and to develop psychiatric disorders.25 Premenstrual dysphoric disorder (PMDD) and the milder premenstrual syndrome (PMS), migraines and even catamenial epilepsy are importantly involved with ALLO and THDOC.[xxxiii] [xxxiv] [xxxv]
The effects of ALLO and THDOC are modified by counteracting neurosteroids (e.g. 3β-THP; DHEA-S and pregnenolone-S) 26 [xxxvi] [xxxvii] and opposing effects of neuro-active steroids.24 [xxxviii] [xxxix] [xl] Ultimately, neurosteroids are deactivated and degraded by local enzymes.25
It must be noted that receptors themselves can adaptively change their structure, function and even numbers (subunit composition, phosphorylation state and population) in response to different amounts and types of stimulation by steroids or drugs.[xli] [xlii] These changes cannot be measured but their effects explain some clinical “paradoxes,” such as the role of neurosteroids in postpartum depression.
Why does Case 1 have too much 5α-R activity?
Insulin resistance is the likely answer to this question. This is certainly the case amongst women with polycystic ovary syndrome (PCOS), of which insulin resistance is an hallmark.[xliii] [xliv] Like Case 1, women with PCOS overproduce 5α-R – largely type-1 – and this is a “key” cause of their diagnostic virilization.[xlv] Indeed, the similarly diagnostic infertility of PCOS also results from excessive 5α-R production in the ovary.45[xlvi] [xlvii] Now hear this: High insulin causes excessive 5α-R activity.
A number of reports convincingly correlate elevated insulin with higher 5α-R – and have even identified 5α-reductase as a treatment-target.20 [xlviii] [xlix] A definitive study incubated cultured human ovarian granulosa cells with insulin in varying concentrations: In a dose-dependent manner, insulin stimulates the ovary to increase 5α-R production.46 Repeating for emphasis: As more insulin is applied, more 5α-reductase is made.
Many women fall victim to this. It is estimated up to 18% of Western women have PCOS.[l] Far more women are insulin-resistant and therefore have elevated insulin – up to 40% of the U.S. population and over 50% of some ethnic groups.[li] We shall now see why this is associated with the incidence in some 3-8% of women of PMDD and up to 25% with the milder version, PMS.27
Why do luteal levels of progesterone bother women like Case 1?
The answer is founded upon this basic fact: Too much ALLO causes distressing neurological symptoms. Most women tolerate even robust amounts of progesterone (e.g. during luteal phase or pregnancy) without problem because their normal 5α-R limits its conversion to ALLO. However, when women like Case 1 make too much 5α-R, their ALLO production is now restricted only by the availability of progesterone. Hence, the occasions of high progesterone in luteal phase or pregnancy can become associated with distressing neurological symptoms.
An apparent paradox must be acknowledged: Research in rodents and the observations of men indicate that low ALLO is associated with anxiety behavior and increased pain perception.26 [lii] However, further valid studies show excessive ALLO also is related to similar neurological symptoms. Understanding now that the response to neurosteroids is bi-phasic, an old puzzle can be solved: For decades, the obvious association of sex hormone changes with PMDD, PMS and postpartum depression was studied – and the only outcome was a “frustrating lack of evidence.” [liii] The neurosteroids offer an explanation.
Women with these problems overly produce 5α-R, thus excessively make ALLO when progesterone is available. Researchers able to measure ALLO find women with PMDD have higher ALLO compared to progesterone (ALLO/progesterone ratio)53 and on taking oral progesterone, these women produce the mostALLO of anyone.[liv] Their blood levels of ALLO consistently reflect those of progesterone: Lowest in follicular and highest in luteal (when symptoms emerge) and then low again at the onset of menses (when symptoms remit).[lv] [lvi] Furthermore, symptoms are absent when progesterone fails to rise during anovulatory cycles[lvii] [lviii] and taking progesterone can worsen their symptoms.53 We are now prepared to consider treatment for these problems.
Case 2: Treatment of premenstrual dysphoric disorder
This woman is a 38 year old, 5’ 9” 140 lb. nulligravida with PMDD. Although a Varsity athlete, she needed antibiotics for adolescent acne. In her early twenties, she was diagnosed with “atypical” or type-2 bipolar disorder. She has taken many psychoactive medications, including SSRI drugs, clonazepam, bupropion and divalproex – often in combination but affording little relief. Her family history is positive for PTSD and alcoholism (1st Marines WWII), cardiovascular disease, PCOS, depression and hypothyroidism.
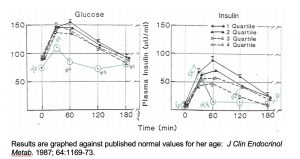
In her mid-20s, incapacitating premenstrual dysphoria and severe dysmenorrhea forced her to drop out of Harvard graduate school. She had noticeable alopecia related to her cycle. Evaluation (July 2000) showed free T3 at the first-centile of the reference interval. Her 24 hour-urine steroids were consistent either with stress and adrenal fatigue or 11β-HSD dysfunction (her 5α and 5β-reductase products balanced normally). Her oGTT with insulin was abnormal, with but little glycemic response and insulin resistance revealed by the areas under the curves (AUC) (Figure 3).
Figure 3: Case 2 oral glucose tolerance test with insulin, July 2000; taking divalproex.
Fifteen years later (August 2015), her PMDD still bothered her greatly. One week every month found her disabled by fatigue; pain; mood swings and irritability – straining the relationship with her co-habiting Significant Other. She said: “I’ve tried all the anxiety meds, all the Psych. meds” and none helped much. She had tried “every” oral contraceptive and all seemed too strong: “They flipped me out” and made her feel angry.
After an appropriate informed consent-talk (Doc trained as a surgeon), she began a 5α-reductase inhibitor, Saw Palmetto Extract (SPE) 320 mg twice-daily. Asked about the results, she effused: “It was amazing!” Within two weeks, she felt happier and “more level.” Through the entire next menstrual cycle, she had remarkably reduced physical and emotional distress that had characterized her previous 15-plus years.
After taking SPE 320 mg bid for 5 months, she estimated she had 50% relief of her overall PMDD and 70% improved mood. Some symptoms persisted at that dose of Saw Palmetto: She missed one work-day each month due to fatigue, not pain; she slept nearly all that day. She stopped this herbal drug for one month and had “horrible” PMDD!
She was happy to learn she could increase her dose to 450 mg BID, which improved her relief to 75%. She still misses one day of work not every month but the majority. Her Significant Other commented he no longer has to “move out 1 week every month.” In fact, they are planning to marry. She knows saw palmetto is contraindicated in pregnancy, so has no plan ever to become pregnant. Her evaluation: Saw palmetto is “the best thing ever.”
Conventional endocrine treatments for PMDD/ PMS
Treatment with oral contraceptives is commonly employed to relieve menstrual problems, which are significantly (up to 95%) associated with insulin resistance.1 [lix] Doc originally thought the OCP simply suppressed the ovaries but the progestin it contains indeed acts differently than progesterone.[lx] Progestins inhibit the normally acquiescent 3α-HSD, so that this enzyme assumes from 5αR the role of rate-limiting “barrier” and limits ALLO production.[lxi] Progestins even alter GABAA-receptors.61 [lxii] This treatment was satisfactory for Case 1 but did not help Case 2.
In order to relieve PMDD/ PMS among PCOS patients while maintaining fertility, insulin resistance is addressed. In addition to diet (per Case 1), metformin is commonly used.[lxiii] This drug improves insulin-sensitivity and reduces long-term complications of insulin resistance while remaining weight-neutral.[lxiv] However, metformin is not reliably effective in controlling the 5α-R related problem of hirsutism.[lxv]
Novel treatment: 5α-reductase blockade
The trail of evidence impressively implicates insulin-induced overproduction of 5α-R and thereby ALLO with PMDD and PMS. If 5α-reductase excess causes PMDD/ PMS, treatment with 5α-R blockers offers an effective alternative, barring pregnancy. Indeed, the relationship between 5α-R and PMDD has been proven to be cause-and-effect by a lovely small study, the first of its kind.[lxvi] In this NIH-supported report, the drug dutasteride – which blocks all three 5α-R isoforms and is not approved for use in women – both inhibited the luteal-phase increase of ALLO and prevented symptoms of PMDD in six of the eight women treated.
5α-Reductase blockers
Two classes of agents block the action of 5α-R.66 First are steroidal agents, the drugs finasteride and dutasteride. Both have long been approved for men’s use in benign prostatic hypertrophy and the former also for male-pattern hair loss.[lxvii] Dutasteride is now shown to help women with PMDD, though it is not approved for their use and it has a rather remarkably long working half-life of 5 weeks.66 Although effective in rodents,[lxviii] [lxix] finasteride should not be used to treat women’s PMDD, because it cannot block the version of type-1 5α-R active in human brains.[lxx]
The second class, non-steroidal 5α-R blockers may offer advantages in effectiveness, availability and price. This is a broad and diverse group, including Saw Palmetto and Reishi (“Chinese mushrooms”)[lxxi] and also benzoquinolones; nonsteroidal aryl acids; butanoid acid derivatives; polyunsaturated fatty acids (esp. linolenic acid); zinc and green tea.6
What is saw palmetto and how could it help PMDD?
Saw Palmetto (Serenoa repens) is a dwarf palm indigenous to the south-eastern U.S.[lxxii] Its berries yield the medicinal extract – of which the active ingredients are various fatty acids.[lxxiii] [lxxiv] Saw Palmetto has long been an American folk medicine, used as a “nerve sedative” and for its effects on reproductive organs and urinary symptoms.[lxxv] [lxxvi] These statements could describe treatment for PMDD/ PMS.
The fatty acids in Saw Palmetto Extract (SPE) vary in potency by tissue type[lxxvii] and individual 5α-R isoform.74 SPE Inhibits both types 1 and 2 5α-reductase.77 [lxxviii] Its action on type-1 5α-R is most relevant for PMDD, since the human brain uses this isoform.78
Saw Palmetto extract relieved Case 2’s PMDD because it inhibits human type 1 5α-R very well – some ten times-more potently than does finasteride.[lxxix] In comparison, dutasteride is said to block human type 1 5α-R 100 times-more potently than finasteride.[lxxx]
The side effects of Saw Palmetto extract are few: A meta-analysis of blinded studies found the withdrawal rate from SPE was 9%, midway between that from finasteride (11%) and from placebo (7%).[lxxxi] Its most significant side-effect may be a reduced libido,39 although various others have been reported.73 Healthy women make little 5α-R and congenitally deficient women are little-effected, so one might expect its inhibition is not objectionable. However, it is absolutely contraindicated in pregnancy and nursing a child: Its effects could mimic the male pseudo-hermaphroditism of type-2 5α-R deficiency.
Saw Palmetto Extract has few human hepatic-drug interactions,[lxxxii] though some have been suggested.[lxxxiii] Due to effects on cortisol and neurosteroids, it seems prudent to monitor liver insulin sensitivity and blood lipid levels during treatment with any 5α-R blocker.[lxxxiv] [lxxxv] A knowledgeable reviewer endorses SPE as safe for long-term use.73
Progesterone treatment for PMDD/ PMS
The informed reader is excused for resisting the statement: “Too much progesterone causes PMDD/ PMS when added to excessive 5α-R.” Many practitioners understand that PMDD/ PMS is caused by “estrogen dominance,” meaning too little progesterone.[lxxxvi] After all, Dalton proved women with PMS respond to added progesterone [lxxxvii] – but relevant for this paper, she later stated doses had to be of pharmacological-strength (supra-physiological) and that testing blood progesterone revealed nothing significant.[lxxxviii] [lxxxix]
Dalton’s methods are still applied. At (Integrative) meetings, Practitioners state some patients require 400 mg (or even more) oral progesterone daily to relieve their PMDD/ PMS symptoms. Blood tests show the resulting progesterone levels are consistent with advanced pregnancy… the term “Progesterone Resistance” is applied. These observations are valid and it seems 5α-R excess and ALLO can explain this “resistance,” also.
There is a bi-modal relationship between serum ALLO and women’s mood.
In a well-designed study, investigators found serum ALLO values between 1.5 and 2.0 nmol/L are associated with significantly negative mood… but women’s mood is good when concentrations are either lower or higher(Figure 4) – a bi-modal effect.[xc] The researchers had given menopausal women various doses of progesterone orally and so-doing, also found the progesterone blood levels producing the “dysphoric” amounts of ALLO are those of normal endogenous luteal phase. Either lower or significantly greater blood levels were better tolerated. Upholding Dalton, the fact is: The ill-effects of “too much” progesterone producing “too much” ALLO can be overcome by giving more progesterone (Figure 4). There are several possible explanations but none are proven.
Figure 4: The biphasic association of blood ALLO concentration and mood in women

These biphasic effects of ALLO may be explained by invoking altered receptor function, perhaps an acute adaptation to “saturation” by ALLO. Some researchers strongly advocate such a mechanism.27 It is possible that receptor adaptation could explain catamenial seizures,[xci] menstrual migraine and perhaps postpartum depression with its incomplete response to therapeutic progesterone.53
Alternately, the biphasic response could be related to a yin/ yang balancing-act of ALLO with progesterone. Functional MRI studies correlated significant variations in amygdala activity with changes in the ratio of ALLO-to-progesterone. Specifically, the effects of a lower ALLO/ progesterone ratio resembled benzodiazepine treatment, while higher ratios mimic anxiety reactions.27 How is this balance altered?
Simply, the ALLO/ progesterone ratio depends upon two variables: The activity of 5α-R and availability of progesterone substrate. Higher, luteal levels of progesterone and increased 5α-reductase combine to produce elevated ALLO and a high ALLO/ progesterone balance. When more progesterone is added, by Dalton’s method for instance, the maximum reaction rate (Vmax) of 5α-R may be exceeded, whereupon the ALLO/ progesterone ratio is expected to decline towards normal.
Are pharmacological doses of progesterone desirable?
With this knowledge of 5α-R and neurosteroids, which Dalton lacked, reconsidering her progesterone treatment introduces concerns about the disadvantages of supra-physiological dosing. High, pregnancy-levels of ALLO alter GABAA-receptors; it is appropriate to feel apprehensive regarding “withdrawal” symptoms – like postpartum depression, seizures or migraines. Secondly, progesterone is a hormone with many effects (such as weight gain), which high levels may express in excess. Thirdly, progesterone can be metabolized into other steroids, including via the “backdoor pathway” to DHT. When fertility is not desired, blocking 5α-R is an attractive alternative.
Case 3: Dysmenorrhea and 5alpha-reductase
In 2005, this 34 year-old, 5’ 8” and 170 lbs. nulligravida woman had monthly severe dysmenorrhea attributed to her laparoscopically-proven endometriosis (at tubal ligation). She was able to continue working only by taking celecoxib 200 mg in doses of 6-8 daily for 2-3 days. Her family history is positive for type-2 diabetes; obesity; hypertension; stroke and heart attack. She has Hashimoto’s disease with dysfunctional deiodination that responded to desiccated thyroid, USP but not to levothyroxine.
She had earlier been evaluated for symptoms considered “hormone imbalance” and her 24 hour-urine adrenal steroid profile showed excessive 5α-R in the high androsterone to etiocholanolone balance. This was validated by her subsequent response to an unwise (in retrospect) supplementation with DHEA 12.5 mg twice-daily: Her facial acne worsened and hypogastric midline hirsutism appeared. Prior to stopping the DHEA, she collected a second 24 hour-urine which proved her excessive 5alpha-reduction produced high androsterone values.
She later performed a 4 hour-oral glucose tolerance test with insulin to evaluate her insulin resistance. Her glycemic counter-regulatory response was poor – indeed, she became hypoglycemic to 49 at two hours – and her areas under the curves prove insulin resistance (Figure 5). Dietary and lifestyle measures were recommended.
Figure 5: Case 3 oral glucose tolerance test with insulin, March 2003

In 2005, her symptoms were consistent with “estrogen dominance” but blood tests instead showed estradiol was low relative to progesterone – in two consecutive menstrual cycles and during her symptoms of PMS! Did she have “progesterone dominance?” Or, was her PMS and subsequent severe dysmenorrhea somehow related to her demonstrated excessive 5α-R activity, acne and sensitivity to the virilizing effects of DHEA? Doc guessed “yes” and thinking too much DHT might be to blame, suggested Saw Palmetto – the right thing for the wrong reason.
Case 3 started Saw Palmetto extract 160 mg twice-daily and at her next menses two weeks later, breast tenderness was reduced but her menses were heavier, longer and cramping was worse. By the second and third months, SPE treatment clearly was helping: She estimated her cramps were reduced by 75%; her flow was lighter and shorter; her cycle had lengthened from 26 to 28 days and her breast tenderness was 75% better.
As the “index patient” for this treatment, she agreed to stop SPE – briefly. Her first month saw a 25 day cycle with heavier bleeding and “hideous cramps.” Her breast pain also returned to baseline severity and she resumed saw palmetto.
Taking 320 mg Saw Palmetto Extract twice-daily, she needs only 400 mg ibuprofen once on day 2 to control her pain. However, her best SPE dose is 450 mg twice-daily, on which she needs no analgesics at all. Her flow is less on 450 mg bid than with the lower dose and her cycle is now consistently 27-28 days. Her sensation of pelvic inflammation is relieved completely by the higher dose. She reports: “That sh-t is fabulous.”
Her androgenic signs were most appreciated after their remission with Saw Palmetto: On the higher dose, she has no facial acne and her hair is thicker in front (father and maternal grandfather both were bald). She realized that the annoying axillary odor for which she had consulted two dermatologists has disappeared with SPE. Why? Apocrine axillary secretions contain pheromones – “fragrant” 5α-reduced androgens including her demonstrably excessive androsterone and androstenone.[xcii] [xciii]
She offers this caution to women: Take SPE without interruption! When she stopped it for 3 weeks, “all hell broke loose” with her next menses; she was “right back where I started.” Doc adds a second warning: It cannot be taken when breast-feeding or during a pregnancy, else Junior may be born without his “pieces-parts.”
5alpha-Reductase and pain
Pain was Case 4’s primary complaint and it responded dramatically to the 5α-R inhibitor. In addition to effecting mood, neurosteroids alter pain transmission in the spinal cord and in neuropathic conditions.[xciv] Here again, we see the “biphasic response,” because normal amounts of ALLO have analgesic and neuro-protective effects.61 Indeed, among male veterans who served in war zones, low ALLO blood levels are significantly associated with low-back and chest pain.52
However, women in luteal phase – when progesterone far exceeds men’s – have increased sensitivity to pain that correlates with higher ALLO concentrations.[xcv] The authors of this study invoked the biphasic effect of ALLO as they observed that very high ALLO may be analgesic (as in pregnancy) but luteal phase ALLO levels can be associated with heightened sensitivity to pain.
Case 4: Migraine headaches
Case 4 is a woman with migraine headaches and positive family history for type-2 diabetes. She was a sickly child with allergies and other issues including hypoglycemia. She got migraine headaches with her menses. Progestin did not help these – nor did it help her premenstrual dysphoria, clumsiness and dysmenorrhea. Ultrasound demonstrated multiple ovarian cysts and endometriosis was suspected. She became hypothyroid from Hashimoto’s disease; her symptoms responded better to desiccated thyroid, USP than to levothyroxine treatment.
In her early 40s, a 24 hour-urine adrenal steroid profile confirmed excessive 5α-R activity with high androsterone/ etiocholanolone ratio. The “Yeast Connection” diet became the most successful she had ever tried and she lost an almost worrisome amount of weight. Eating this low glycemic-index, low insulinemic-index and slowly accessible-glucose (no-sugar, no-starch and no-fruit) diet, she had “the best period of my life” with no PMS whatsoever. With incomplete adherence, though, PMS symptoms relapsed.
Her breast cancer was found in 1997: Both estrogen and progesterone receptors were positive. Post-operative chemotherapy hastened her menopause. She developed hot flashes; felt stressed and fatigued; spacey and forgetful and she couldn’t sleep. Polyarthralgia began and she lost bone density – taking raloxifene helped only the latter. Persisting insomnia became a real problem. In 2001, she briefly tried HRT with estriol and progesterone cream based on salivary tests but felt neither well nor safe using them. She began cultivating black cohosh in her back yard.
Seventeen years post-breast cancer, she used estradiol vaginal suppositories (Vagifem®). Her insomnia persisted, featuring prolonged 3 AM awakenings. Her energy was variably poor. Migraine-severity headaches with nausea occurred at waking twice-monthly and could last for days… for these, she needed opiate medication. More frequently, milder and brief headaches began later in the day. A variety of antidepressants had not improved her mood, just made her feel “fuzzy.” Her therapist said she should consider starting HRT.
On Doc’s evaluation, her diet was “clean.” Her adrenals seemed adequately supported and “natural” thyroid replacement gave her good thyroid blood levels. Blood tests (8.4.2014) showed estradiol = 7.1 pg/mL (menopause reference interval < 55); progesterone 0.2 ng/mL (0.1-0.8) and total testosterone 23 ng/dL (3-41). They had a detailed informed-consent talk about the risks and benefits of resuming HRT in her circumstances. After several months of deliberation, she chose to try transdermal estradiol, balanced with progesterone derived from oral pregnenolone – with the goal of recapitulating mid-follicular blood levels to improve her symptoms.
This was achieved with Vivelle Dot® estradiol patch 0.0375 mg twice-weekly; pregnenolone 20 mg at bedtime and neonatal bovine adrenal cortex. Her sleep was significantly improved but her headaches worsened. She awoke with pulsating retro-orbital pain associated with nausea – as formerly associated with her menstrual cycles – on average twice and up to four times-weekly. The pain could last 3 days; it might be aborted if she promptly took sumatryptan with ibuprofen. During this time, her blood tests showed estradiol= 49.4 pg/mL; progesterone 0.7 ng/mL; tTest 18 ng/dL and free testosterone 1.6 pg/mL (0.0-4.2).
Doc recommended a 5alpha –reductase blocker and she preferred Saw Palmetto. He offered a few samples of his wife’s 450 mg and these were too strong, upsetting her stomach. She switched to 160 mg once-daily and soon reported 5 consecutive pain-free days. After 3 months, Saw Palmetto Extract 160 mg 3 times-daily was relieving about two thirds of her migraines – in fact, headaches were better than ever.
Further clinical applications
The experiences of Cases 1 and 4 offer another useful lesson: The design of women’s hormone replacement therapy (HRT) should consider their history for increased 5α-R and accommodate for it. A number of menopausal women shun progesterone treatment because it causes headaches and other symptoms. Doc sets therapeutic goals at follicular blood levels, for progesterone as well as estradiol – thereby avoiding the risk of reproducing PMS symptoms from luteal progesterone levels. Oral pregnenolone can easily and inexpensively achieve progesterone values about 1.0 ng/mL to balance follicular estradiol levels. It is also theoretically possible pregnenolone sulfate may balance ALLO – but clearly, it does not always.
Although even a modest dose of progesterone or pregnenolone causes some women distress, Doc learned these women often can tolerate only a small initial dose of SPE – or else their pain and symptoms worsen. In this way they seem to resemble the male Veterans cited above.52 By slowly increasing their doses of both pregnenolone and Saw Palmetto Extract, they may achieve follicular progesterone values without distress. The requisite SPE dose is usually 320 mg twice-daily or less.
Can induction of 5alpha-reductase in the skin effect transdermal progesterone?
The companion to this article described a man who suffered a spectacular failure of his transdermal testosterone replacement after an overdose of the applied hormone caused his skin to make 5alpha-reductase excessively. Blood tests showed the enzyme destroyed his testosterone by converting it to DHT.4 Progesterone also is a substrate for 5α-R. It is logical to suspect that in a similar fashion, topical progesterone may stimulate women’s skin to over-produce 5α-R.
Doc has heard reports of women who after prolonged use of topical progesterone could “no longer absorb it.” Their skin is said to be “saturated” and no matter how much progesterone they apply (2% cream gives 20 mg progesterone per gram and 10% delivers 100 mg/gm), no increase in salivary or blood progesterone can be measured.
Long puzzled by this, Doc can now offer an hypothesis: Topical progesterone can induce skin to make 5α-R – and if “men’s Case 1” is a guide, the more progesterone is applied, the faster 5α-R is induced. The skin is not “saturated” but is equipped to rapidly destroy absorbed progesterone – by 5alpha-reduction. Progesterone isabsorbed – and killed. If so, then women, like men, have a “first-pass effect” in both the skin and the liver.
Is 5α-R involved with hypoglycemia?
For some years, the American Academy of Environmental Medicine’s New Endocrinology course has presented a peculiar observation: A group of young women with symptoms of insulin resistance and mood disturbances from mild anhedonia to type-2 bipolar disorder show a peculiar response to oral GTT with insulin tests: They have a blunted, sometimes virtually absent glycemic response with just enough excessive insulin response to indicate its greater AUC.
After reviewing these test results from Cases 2 and 3, Doc recalled Case 1’s hypoglycemia and went to Pub Med. These “flat” glucose responses may be related to dysfunctional hypothalamic GABAA-receptor function, which impairs the counter-regulatory glycemic response.28 29
Finally, is there a link between neurosteroids and fibromyalgia? Certainly, neurosteroids are involved with pain transmission and fibromyalgia is properly described as central nociceptive hyperesthesia – heightened perception of pain within the central nervous system. Fibromyalgia is strongly associated with elevated cytokines and the Pub Med search field “neurosteroids AND cytokines” yields over 30,000 citations. Lastly, the drug most commonly given for fibromyalgia is gabapentin… the GABA receptor, right? Further investigations into a possible connection between 5α-R and fibromyalgia may be productive – as might treatment trials with saw palmetto extract for selected patients.
Conclusion
5α-R and its neurosteroid products are significant for some of our patients, as indicated by these “anecdotal” case studies and a small but high-quality NIH-sponsored trial using a 5α-reductase inhibitor (dutasteride).66 When conventional treatments for problems related to 5α-R excess such as PMDD; postpartum depression; catamenial seizures; menstrual migraine and alopecia, acne and hirsutism are ineffective (or unsuitable orsimply not the patient’s preference), the use of a 5α-R blocker can be considered if fertility is not desired.
Saw palmetto in this century has been considered a “man’s treatment” but it offers more. It can be used successfully for PMDD/ PMS and dysmenorrhea –perhaps other neurosteroid-related problems as well – but do so wisely: It is contraindicated in pregnancy and nursing.
References
[i] Fernandes AR, de Sá Rosa e Silva AC, Romão GS, et al. Insulin resistance in adolescents with menstrual irregularities. J Pediatr Adolesc Gynecol. 2005 Aug; 18(4):269-74.
[ii] Chen C, Doherty JA, Lewis SK, et al. Insulin-like growth factor-I, insulin-like growth factor binding protein-3 and the risk of fibrocystic breast conditions among Chinese women. Int J Cancer. 2006 May 1; 118(9):2303-9.
[iii] Verberne AJ, Sabetghadam A, Korim WS. Neural pathways that control the glucose counterregulatory response. Front Neurosci. 2014 Feb 26;8:38. doi: 10.3389/fnins.2014.00038. PMID: 24616659. Free PMC Article.
[iv] McDaniel AB. The clinical importance of 5alpha-reductase in human health and pathology, part 1: Men, testosterone replacement and stress. Townsend Letter. 2016 Dec. In press.
[v] Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian J Androl. 2016 May-Jun; 18(3):435-40. doi: 10.4103/1008-682X.173932. PMID: 26908066. Free PMC Article.
[vi] Azzouni F, Godoy A, Li Y, Mohler J. The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Adv Urol. 2012; 2012:530121. doi: 10.1155/2012/530121. PMID: 22235201. Free PMC Article.
[vii] Saartok T, Dahlberg E, Gustafsson JA. Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984 Jun; 114(6):2100-6. PMID: 6539197.
[viii] Endocrine Services, LabCorp USA. Laboratory Corporation of America.
[ix] Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25-61. PMID: 7979239.
[x] Uemura M, Tamura K, Chung S et al. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008 Jan; 99(1):81-6. PMID: 17986282. Free full text.
[xi] Cantagrel V, Lefeber DJ, Ng BG et al. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010 Jul 23; 142(2):203-17. doi: 10.1016/j.cell.2010.06.001. PMID: 20637498. Free PMC Article.
[xii] Kenyon P. Sex and gender: nature or nurture? http://www.flyfishingdevon.co.uk/salmon/year1/psy128psychosexual_differentiation/sexdiff.htm. Accessed 9.15.2016.
[xiii] Katz MD, Cai LQ, Zhu YS et al. The biochemical and phenotypic characterization of females homozygous for 5 alpha-reductase-2 deficiency. J Clin Endocrinol Metab. 1995 Nov; 80(11):3160-7. PMID: 7593420.
[xiv] Vassiliadi DA, Barber TM, Hughes BA et al. Increased 5 alpha-reductase activity and adrenocortical drive in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009 Sep; 94(9):3558-66. doi: 10.1210/jc.2009-0837. PMID: 19567518.
[xv] Glintborg D, Hermann AP, Hagen C et al. A randomized placebo-controlled study on the effects of pioglitazone on cortisol metabolism in polycystic ovary syndrome. Fertil Steril. 2009 Mar; 91(3):842-50. doi: 10.1016/j.fertnstert.2007.12.082. PMID: 18402944.
[xvi] Genova Diagnostics Asheville, NC. https://www.gdx.net/product/complete-hormones-test-urine Accessed 6.30.2016.
[xvii] Meridian Valley Lab Tukwila, WA. http://meridianvalleylab.com/wp-content/uploads/2014/07/Adrenal-Profile.pdf Accessed 6.28.2016.
[xviii] Rižner TL, Penning TM. Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids. 2014 Jan; 79:49-63. doi: 10.1016/j.steroids.2013.10.012. PMID: 24189185. Free PMC Article.
[xix] Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004 Nov; 15(9):432-8. PMID: 15519890.
[xx] Crowley RK, Hughes B, Gray J et al. Longitudinal changes in glucocorticoid metabolism are associated with later development of adverse metabolic phenotype. Eur J Endocrinol. 2014 Oct; 171(4):433-42. doi: 10.1530/EJE-14-0256. PMID: 24986533. Free full text.
[xxi] Armandari I, Hamid AR, Verhaegh G, Schalken J. Intratumoral steroidogenesis in castration-resistant prostate cancer: a target for therapy. Prostate Int. 2014 Sep; 2(3):105-13. doi: 10.12954/PI.14063. PMID: 25325021. Free PMC Article.
[xxii] Nixon M, Upreti R, Andrew R. 5α-Reduced glucocorticoids: a story of natural selection. J Endocrinol. 2012 Feb; 212(2):111-27. doi: 10.1530/JOE-11-0318. PMID: 21903862. Free full text.
[xxiii] Melcangi RC, Giatti S, Garcia-Segura LM. Levels and actions of neuroactive steroids in the nervous system under physiological and pathological conditions: Sex-specific features. Neurosci Biobehav Rev. 2016 Aug; 67:25-40. doi: 10.1016/j.neubiorev.2015.09.023. PMID: 26657814.
[xxiv] Melcangi RC, Panzica GC. Allopregnanolone: state of the art. Prog Neurobiol. 2014 Feb; 113:1-5. doi: 10.1016/j.pneurobio.2013.09.005. PMID: 24121112.
[xxv] Gunn BG, Cunningham L, Mitchell SG et al. GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front Neuroendocrinol. 2015 Jan; 36:28-48. doi: 10.1016/j.yfrne.2014.06.001. PMID: 24929099. Free PMC Article.
[xxvi] Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010; 186:113-37. doi: 10.1016/B978-0-444-53630-3.00008-7. PMID: 21094889. Free PMC Article.
[xxvii] Bäckström T, Bixo M, Johansson M et al. Allopregnanolone and mood disorders. Prog Neurobiol. 2014 Feb; 113:88-94. doi: 10.1016/j.pneurobio.2013.07.005. PMID: 23978486.
[xxviii] Chan O, Paranjape S, Czyzyk D et al. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes. 2011 May; 60(5):1582-9. doi: 10.2337/db10-1579. PMID: 21411513. Free PMC Article.
[xxix] Zhu W, Czyzyk D, Paranjape SA et al. Glucose prevents the fall in ventromedial hypothalamic GABA that is required for full activation of glucose counterregulatory responses during hypoglycemia. Am J Physiol Endocrinol Metab. 2010 May; 298(5):E971-7. doi: 10.1152/ajpendo.00749.2009. PMID: 20304763. Free PMC Article.
[xxx] Bitran D, Dugan M, Renda P et al. Anxiolytic effects of the neuroactive steroid pregnanolone (3 alpha-OH-5 beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999 Dec 11; 850(1-2):217-24. PMID: 10629767.
[xxxi] Carboni E, Wieland S, Lan NC, Gee KW. Anxiolytic properties of endogenously occurring pregnanediols in two rodent models of anxiety. Psychopharmacology (Berl). 1996 Jul; 126(2):173-8. PMID: 8856837.
[xxxii] Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996 Dec; 15(6):533-40. PMID: 8946427. Free full text.
[xxxiii] Finocchi C, Ferrari M. Female reproductive steroids and neuronal excitability. Neurol Sci. 2011 May; 32 Suppl 1:S31-5. doi: 10.1007/s10072-011-0532-5. PMID: 21533709.
[xxxiv] Dazzi L, Serra M, Seu E et al. Progesterone enhances ethanol-induced modulation of mesocortical dopamine neurons: antagonism by finasteride. J Neurochem. 2002 Dec; 83(5):1103-9. PMID: 12437581. Free full text.
[xxxv] Engel SR, Grant KA. Neurosteroids and behavior. Int Rev Neurobiol. 2001; 46:321-48. PMID: 11599304.
[xxxvi] Genazzani AR, Bernardi F, Monteleone P et al. Neuropeptides, neurotransmitters, neurosteroids, and the onset of puberty. Ann N Y Acad Sci. 2000; 900:1-9. PMID: 10818386.
[xxxvii] Maitra R, Reynolds JN. Modulation of GABA(A) receptor function by neuroactive steroids: evidence for heterogeneity of steroid sensitivity of recombinant GABA(A) receptor isoforms. Can J Physiol Pharmacol. 1998 Sep; 76(9):909-20. PMID: 10066142.
[xxxviii] Sarkar J, Wakefield S, MacKenzie G et al. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011 Dec 14; 31(50):18198-210. doi: 10.1523/JNEUROSCI.2560-11.2011. PMID: 22171026. Free PMC Article.
[xxxix] King SR. Emerging roles for neurosteroids in sexual behavior and function. J Androl. 2008 Sep-Oct; 29(5):524-33. doi: 10.2164/jandrol.108.005660. PMID: 18567641. Free full text.
[xl] Purdy RH, Morrow AL, Moore PH Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991 May 15; 88(10):4553-7. PMID: 1852011. Free PMC Article.
[xli] Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA(A) Receptor Interactions: A Focus on Stress. Front Neurosci. 2011 Dec 5; 5:131. doi: 10.3389/fnins.2011.00131. PMID: 22164129. Free PMC Article.
[xlii] Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007 Oct; 116(1):20-34. PMID: 17531325.
[xliii] Vassiliadi DA, Barber TM, Hughes BA et al. Increased 5 alpha-reductase activity and adrenocortical drive in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009 Sep; 94(9):3558-66. doi: 10.1210/jc.2009-0837. PMID: 19567518.
[xliv] Fassnacht M, Schlenz N, Schneider SB et al. Beyond adrenal and ovarian androgen generation: Increased peripheral 5 alpha-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003 Jun; 88(6):2760-6. PMID: 12788885.
[xlv] Jakimiuk AJ, Weitsman SR, Magoffin DA. 5alpha-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1999 Jul; 84(7):2414-8. PMID: 10404813.
[xlvi] Kayampilly PP, Wanamaker BL, Stewart JA et al. Stimulatory effect of insulin on 5alpha-reductase type 1 (SRD5A1) expression through an Akt-dependent pathway in ovarian granulosa cells. Endocrinology. 2010 Oct; 151(10):5030-7. doi: 10.1210/en.2010-0444. PMID: 20810561. Free PMC Article.
[xlvii] Magoffin DA. Ovarian enzyme activities in women with polycystic ovary syndrome. Fertil Steril. 2006 Jul; 86 Suppl 1:S9-S11. DOI: 10.1016/j.fertnstert.2006.03.015. PMID: 16798289.
[xlviii] Baudrand R, Domínguez JM, Carvajal CA et al. Overexpression of hepatic 5α-reductase and 11β-hydroxysteroid dehydrogenase type 1 in visceral adipose tissue is associated with hyperinsulinemia in morbidly obese patients. Metabolism. 2011 Dec; 60(12):1775-80. doi: 10.1016/j.metabol.2011.05.001. PMID: 21704348.
[xlix] Tsilchorozidou T, Honour JW, Conway GS. Altered cortisol metabolism in polycystic ovary syndrome: insulin enhances 5alpha-reduction but not the elevated adrenal steroid production rates. J Clin Endocrinol Metab. 2003 Dec; 88(12):5907-13. DOI: 10.1210/jc.2003-030240. PMID: 14671189.
[l] Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010 Jun 30; 8:41. doi: 10.1186/1741-7015-8-41. PMID: 20591140. Free PMC Article.
[li] Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002 Jan 16; 287(3):356-9. PMID: 11790215.
[lii] Kilts JD, Tupler LA, Keefe FJ et al. Neurosteroids and self-reported pain in veterans who served in the U.S. Military after September 11, 2001. Pain Med. 2010 Oct; 11(10):1469-76. doi: 10.1111/j.1526-4637.2010.00927.x. PMID: 20735755. Free PMC Article.
[liii] Schiller CE, Schmidt PJ, Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berl). 2014 Sep; 231(17):3557-67. doi: 10.1007/s00213-014-3599-x. PMID: 24846476. Free PMC Article.
[liv] Klatzkin RR, Morrow AL, Light KC et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone.Psychoneuroendocrinology. 2006 Nov; 31(10):1208-19. DOI: 10.1016/j.psyneuen.2006.09.002. PMID: 17046166.
[lv] Schmidt PJ, Purdy RH, Moore PH Jr et al. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994 Nov; 79(5):1256-60. DOI: 10.1210/jcem.79.5.7962316. PMID: 7962316.
[lvi] Genazzani AR, Petraglia F, Bernardi F et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998 Jun; 83(6):2099-103. DOI: 10.1210/jcem.83.6.4905. PMID: 9626145.
[lvii] Frye CA. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology. 2009 Dec; 34 Suppl 1:S143-61. doi: 10.1016/j.psyneuen.2009.07.005. PMID: 19656632. Free PMC Article.
[lviii] Andréen L, Nyberg S, Turkmen S et al. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. 2009 Sep; 34(8):1121-32. doi: 10.1016/j.psyneuen.2009.02.003. PMID: 19272715.
[lix] Bouzas IC, Cader SA, Leão L et al. Menstrual cycle alterations during adolescence: early expression of metabolic syndrome and polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2014 Dec; 27(6):335-41. doi: 10.1016/j.jpag.2014.01.002. PMID: 25256874.
[lx] Holtorf K. The bioidentical hormone debate: are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy? Postgrad Med. 2009 Jan; 121(1):73-85. doi: 10.3810/pgm.2009.01.1949. PMID: 19179815.
[lxi] Patte-Mensah C, Meyer L, Taleb O, Mensah-Nyagan AG. Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain. Prog Neurobiol. 2014 Feb; 113:70-8. doi: 10.1016/j.pneurobio.2013.07.004. PMID: 23948490.
[lxii] Porcu P, Mostallino MC, Sogliano C et al. Long-term administration with levonorgestrel decreases allopregnanolone levels and alters GABA(A) receptor subunit expression and anxiety-like behavior. Pharmacol Biochem Behav. 2012 Aug; 102(2):366-72. doi: 10.1016/j.pbb.2012.05.011. PMID: 22634062.
[lxiii] Nestler JE. Metformin in the treatment of infertility in polycystic ovarian syndrome: an alternative perspective. Fertil Steril. 2008 Jul; 90(1):14-6. doi: 10.1016/j.fertnstert.2008.04.073. PMID: 18550055. Free PMC Article.
[lxiv] Cheang KI, Huszar JM, Best AM et al. Long-term effect of metformin on metabolic parameters in the polycystic ovary syndrome. Diab Vasc Dis Res. 2009 Apr; 6(2):110-9. doi: 10.1177/1479164109336050. PMID: 20368201. Free PMC Article.
[lxv] Roth LW, Huang H, Legro RS et al. Altering hirsutism through ovulation induction in women with polycystic ovary syndrome. Obstet Gynecol. 2012 Jun; 119(6):1151-6. doi: 10.1097/AOG.0b013e31825618fb. PMID: 22617579. Free PMC Article.
[lxvi] Martinez PE, Rubinow DR, Nieman LK et al. 5α-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder.Neuropsychopharmacology. 2016 Mar; 41(4):1093-102. doi: 10.1038/npp.2015.246. PMID: 26272051.
[lxvii] Finn DA, Beadles-Bohling AS, Beckley EH et al. A new look at the 5alpha-reductase inhibitor finasteride.CNS Drug Rev. 2006 Spring; 12(1):53-76. DOI: 10.1111/j.1527-3458.2006.00053.x. PMID: 16834758. Free full text.
[lxviii] Lephart ED, Ladle DR, Jacobson NA, Rhees RW. Inhibition of brain 5 alpha-reductase in pregnant rats: effects on enzymatic and behavioral activity. Brain Res. 1996 Nov 11; 739(1-2):356-60. PMID: 8955960.
[lxix] Concas A, Mostallino MC, Porcu P et al. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998 Oct 27; 95(22):13284-9. PMID: 9789080. Free PMC Article.
[lxx] Kenny B, Ballard S, Blagg J, Fox D. Pharmacological options in the treatment of benign prostatic hyperplasia. J Med Chem. 1997 Apr 25; 40(9):1293-315. DOI: 10.1021/jm960697s. PMID: 9135028.
[lxxi] Grant P, Ramasamy S. An update on plant derived anti-androgens. Int J Endocrinol Metab. 2012 Spring; 10(2):497-502. doi: 10.5812/ijem.3644. PMID: 23843810. Free PMC Article.
[lxxii] U.S. National Plant Germplasm System. Taxon: Serenoa repens (W. Bartram) Small. https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?103108. Accessed 9.25.2016.
[lxxiii] Suzuki M, Ito Y, Fujino T et al. Pharmacological effects of saw palmetto extract in the lower urinary tract.Acta Pharmacol Sin. 2009 Mar; 30(3):227-81. doi: 10.1038/aps.2009.1. PMID: 19262550. Free PMC Article.
[lxxiv] Raynaud JP, Cousse H, Martin PM. Inhibition of type 1 and type 2 5alpha-reductase activity by free fatty acids, active ingredients of Permixon. J Steroid Biochem Mol Biol. 2002 Oct; 82(2-3):233-9. PMID: 12477490.
[lxxv] NIH National Center for Complementary and Integrative Health. Saw Palmetto 2012 April. https://nccih.nih.gov/health/palmetto/ataglance.htm. Accessed 9.25.2016.
[lxxvi] Felter, HW. The Eclectic Materia Medica, Pharmacology and Therapeutics. Cincinnati, OH. John K. Scudder. 1922. Reprinted and abridged by Southwest School of Botanical Medicine, Bisbee, AZ http://www.swsbm.com/FelterMM/Felters_Materia_Medica.pdf. Accessed 9.25.2016.
[lxxvii] Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate. 1996 May; 28(5):300-6. DOI: 10.1002/(SICI)1097-0045(199605)28:5<300::AID-PROS5>3.0.CO;2-F. PMID: 8610056.
[lxxviii] Iehlé C, Délos S, Guirou O et al. Human prostatic steroid 5 alpha-reductase isoforms–a comparative study of selective inhibitors. J Steroid Biochem Mol Biol. 1995 Sep; 54(5-6):273-9. PMID: 7577710.
[lxxix] Abe M, Ito Y, Oyunzul L et al. Pharmacologically relevant receptor binding characteristics and 5alpha-reductase inhibitory activity of free Fatty acids contained in saw palmetto extract. Biol Pharm Bull. 2009 Apr;32(4):646-50. PMID: 19336899. Free full text.
[lxxx] Olsen EA, Hordinsky M, Whiting D et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006 Dec; 55(6):1014-23. DOI: 10.1016/j.jaad.2006.05.007. PMID: 17110217.
[lxxxi] Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John’s Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann Intern Med. 2002 Jan 1; 136(1):42-53. PMID: 11777363.
[lxxxii] Markowitz JS, Donovan JL, Devane CL et al. Multiple doses of saw palmetto (Serenoa repens) did not alter cytochrome P450 2D6 and 3A4 activity in normal volunteers. Clin Pharmacol Ther. 2003 Dec; 74(6):536-42. DOI: 10.1016/j.clpt.2003.08.010. PMID: 14663456.
[lxxxiii] Yale SH, Glurich I. Analysis of the inhibitory potential of Ginkgo biloba, Echinacea purpurea, and Serenoa repens on the metabolic activity of cytochrome P450 3A4, 2D6, and 2C9. J Altern Complement Med. 2005 Jun; 11(3):433-9. DOI: 10.1089/acm.2005.11.433. PMID: 15992226.
[lxxxiv] Nasiri M, Nikolaou N, Parajes S et al. 5α-Reductase Type 2 Regulates Glucocorticoid Action and Metabolic Phenotype in Human Hepatocytes. Endocrinology. 2015 Aug; 156(8):2863-71. doi: 10.1210/en.2015-1149. PMID: 25974403. Free PMC Article.
[lxxxv] Hazlehurst JM, Oprescu AI, Nikolaou N et al. Dual-5α-Reductase Inhibition Promotes Hepatic Lipid Accumulation in Man. J Clin Endocrinol Metab. 2016 Jan; 101(1):103-13. doi: 10.1210/jc.2015-2928. PMID: 26574953. Free PMC Article.
[lxxxvi] Lee JR, Hopkins V. What Your Doctor May Not Tell You About Menopause. New York, NY Warner Books; 2004. ISBN: 9780446614955.
[lxxxvii] Greene R, Dalton K. The premenstrual syndrome. Br Med J. 1953 May 9; 1(4818):1007-14. PMID: 13032605. Free PMC Article.
[lxxxviii] Dalton K. The aetiology of premenstrual syndrome is with the progesterone receptors. Med Hypotheses. 1990 Apr; 31(4):323-7. PMID: 2192240.
[lxxxix] Dalton K. Progesterone or progestogens? Br Med J. 1976 Nov 20; 2(6046):1257. PMID: 990874. Free PMC Article.
[xc] Andréen L, Sundström-Poromaa I, Bixo M et al. Allopregnanolone concentration and mood–a bimodal association in postmenopausal women treated with oral progesterone. Psychopharmacology (Berl). 2006 Aug; 187(2):209-21. DOI: 10.1007/s00213-006-0417-0. PMID: 16724185.
[xci] Bäckström T, Andersson A, Andreé L et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci. 2003 Dec; 1007:42-53. PMID: 14993039.
[xcii] Gower DB, Mallet AI, Watkins WJ et al. Capillary gas chromatography with chemical ionization negative ion mass spectrometry in the identification of odorous steroids formed in metabolic studies of the sulphates of androsterone, DHA and 5alpha-androst-16-en-3beta-ol with human axillary bacterial isolates. J Steroid Biochem Mol Biol. 1997 Sep-Oct; 63(1-3):81-9. PMID: 9449209.
[xciii] Bremner EA, Mainland JD, Khan RM, Sobel N. The prevalence of androstenone anosmia. Chem Senses. 2003 Jun;28(5):423-32. PMID: 12826538. Free full text.
[xciv] Coronel MF, Labombarda F, González SL. Neuroactive steroids, nociception and neuropathic pain: A flashback to go forward. Steroids. 2016 Jun; 110:77-87. doi: 10.1016/j.steroids.2016.04.005. PMID: 27091763.
[xcv] Mechlin B, Morrow AL, Maixner W, Girdler SS. The relationship of allopregnanolone immunoreactivity and HPA-axis measures to experimental pain sensitivity: Evidence for ethnic differences. Pain. 2007 Sep; 131(1-2):142-52. DOI: 10.1016/j.pain.2006.12.027. PMID: 17292548. Free PMC Article.
Author bio
Alan McDaniel, MD is a 1977 Tulane medical graduate. He trained in General Surgery and Emergency Medicine before becoming Board-certified in Otolaryngology with sub-specialties in Neurotology and Allergy. He practices privately since a two-year professorship at the University of Louisville.
He has presented to various national meetings, including the American Neurotology Society and the American Academy of Otolaryngology-Head and Neck Surgery and to the World Congress of Otorhinolaryngology. He has been a faculty member for American Academy of Otolaryngic Allergy Basic and Advanced Courses. His two-day course “New Endocrinology” has been presented annually at the American Academy of Environmental Medicine since 2005, to physicians from five continents.
Work with dizziness and allergy in the 1980s led him to seek solutions for Chronic Fatigue Syndrome. In turn, these investigations extended to the endocrine aspects of this and related conditions. Since basic surgical training emphasizes the need to know several alternative approaches to an operation, it was logical for him to study integrative and controversial medical methods. He has endeavoured to understand these in the light of new facts from research, perceiving that Medical history shows innovation begins as a minority opinion.
He is excited that applying new research to patient care offers solutions to many of the chronic and worsening problems that are epidemic in modern society.
Consult your doctor before using any of the treatments mentioned in this article.
Reprinted with permission from the June, 2017 Townsend Letter and Alan B. McDaniel, MD
Learn how you can benefit from more Townsend Letter articles.