Article from Townsend Letter
Metabolic Flexibility – How to Reverse Metabolic Inflexibility to Heal Chronic Disease
by Bonnie Nedrow, ND
Metabolic Flexibility
Metabolic flexibility (MetF) is the ability to rapidly switch between oxidation of carbohydrates and fats based on nutrient availability.1 MetF allows for the storage of fuel when food availability is high and utilization of that stored energy when food availability is poor. This metabolic elasticity is critical to human survival during periods of scarcity and allows for the enjoyment and celebration of food when in abundance.
Insulin is a key player in orchestrating metabolic substrate modulation. When a meal with carbohydrates is eaten, insulin increases, which stimulates oxidation of glucose and storage of both carbohydrates and fatty acids. In a eucaloric state, where food intake matches fuel expenditure, an individual will neither gain nor lose weight. When caloric intake exceeds fuel needs, excess calories are stored as fat to be accessed at a future date to fuel sub-caloric periods of time.
Historically, humans have repeatedly experienced periods of both feasting and famine—to some extent seasonally each year and with extended intervals of low caloric intake during lean years and excess consumption during plentiful years. This variation in caloric intake is also seen on a much smaller scale in the daily cycle of the fasted/fed states. On a daily basis, it is common for people to fast overnight for roughly 10-12 hours: a period longer than can be sustained by glucose metabolism alone. In lean healthy individuals, fatty acid oxidation increases during the overnight fasted state. Following a meal with carbohydrates, these individuals secrete insulin, thereby suppressing fatty acid oxidation and shifting metabolism to primarily glucose oxidation.2 The daily fast/fed cycle in this example demonstrates metabolic flexibility.
When healthy lean people are exposed to prolonged fasting or prolonged exercise, fatty acids and ketones increase and energy is maintained despite an imbalance of fuel intake and expenditure. These are other examples of how MetF maintains optimal function despite environmental unpredictability.
Metabolic Inflexibility
Metabolic inflexibility (MetIF) is a modern malady linked to metabolic syndrome and to many chronic diseases. While it may seem obvious that overconsumption of calorie-dense food is the root cause, it turns out to be much more complicated. Other key contributory insults include poor sleep combined with high stress, environmental toxicants (chemicals that are detrimental to health), poor exercise habits, and a mismatch of activity to circadian rhythms. To reinstate metabolic flexibility in metabolically ill people, all these factors must be addressed.
Insulin, the conductor of metabolic substrate modulation, does not have the same impact on people who are obese and insulin resistant. Contrary to metabolically well people, this metabolic type shows reduced fatty acid oxidation in general, a lack of increase in fat burning during the fasted state, and minimal suppression of fat metabolites with an increase in insulin. These people end up with excess fuel in their blood stream and in storage but lack the ability to easily burn either glucose or fat. They are overweight, fatigued, and inflamed from this excess that does not fuel their body.
Someone who has become metabolically inflexible cannot easily mobilize fat from storage. Instead, they become dependent on frequent meal spacing to stay fueled with carbohydrates. This is driven by neuropenia, a shortage of glucose in the brain, usually due to transient hypoglycemia. It becomes increasingly difficult to stay on a eucaloric diet and nearly impossible to sustain a sub-caloric diet to achieve weight loss and re-establish metabolic flexibility. What was an advantageous metabolism for a world where food security was uncertain has become a curse in a society where abundant food is driving metabolic disease.3
Metabolic Syndrome
As stated earlier, MetIF is associated with metabolic syndrome (MetS): the clinical presentation of a human system on the verge of chronic disease. Diseases associated with this condition include diabetes, obesity, cardiovascular disease, Alzheimer’s, and non-alcoholic liver disease. MetS is defined as elevated blood pressure, truncal weight gain, high fasting glucose, high fasting triglycerides, and low HDL. More recently, elevated liver enzymes, in particular alanine aminotransferase (ALT) and gamma-glutamyl transference (GGT), have been added to the list of biomarkers associated with MetS. Looking beyond the clinical definition, metabolically ill people experience the all-too-common symptoms of weight gain, fatigue, and inflammation throughout their body. According to the National Health and Nutrition Examination Survey (NHANES), the incidence of MetS increased by 35% between 1998-2012 and is a common presentation in a large percentage of patients seeking medical care today.
| Test | Optimal | Metabolic syndrome |
| HDL | >50 | <50 women/<40 men |
| Triglycerides | 50-90 | >150 |
| Fasting glucose | 60-90 | >99 |
| ALT | teens | >23 women/>25 men |
| GGT | teens | >20 |
| Hip/waist | 0.8 or less | >0.85 women/>1.0 men |
| BP | 60-75/120-125 | >80/130 (either value) |
Obesogens
In the past several years, the general knowledge and recognition of the health impacts of endocrine disrupting chemicals has grown. However, the well documented phenomenon of obesogens, a sub-class of endocrine-disruptive compounds, has been slower to be recognized. Given the world-wide epidemic of obesity and the comorbidities associated, there is a growing need for clinicians to incorporate diagnostic and therapeutic modalities aimed at a reduction of both exogenous and endogenous exposures to this class of compounds.
Obesogens are chemicals that interfere with healthy metabolic regulation through promotion of adipogenesis and induction of fat storage. One of the most studied mechanisms for these effects is the peroxisome proliferator-activated receptor PPAR-γ. PPAR-γ programs increased adipocyte number and size in the developmental years as well as promoting lipid accumulation and storage in adults. Additionally, there are many steroidal endocrine disruptive compounds that also induce obesity. Exposure to obesogens during development predisposes an individual to increased susceptibility to these same chemicals at a later date. In this fashion, long-term exposures to obesogens from conception onward drives the obesity epidemic.4
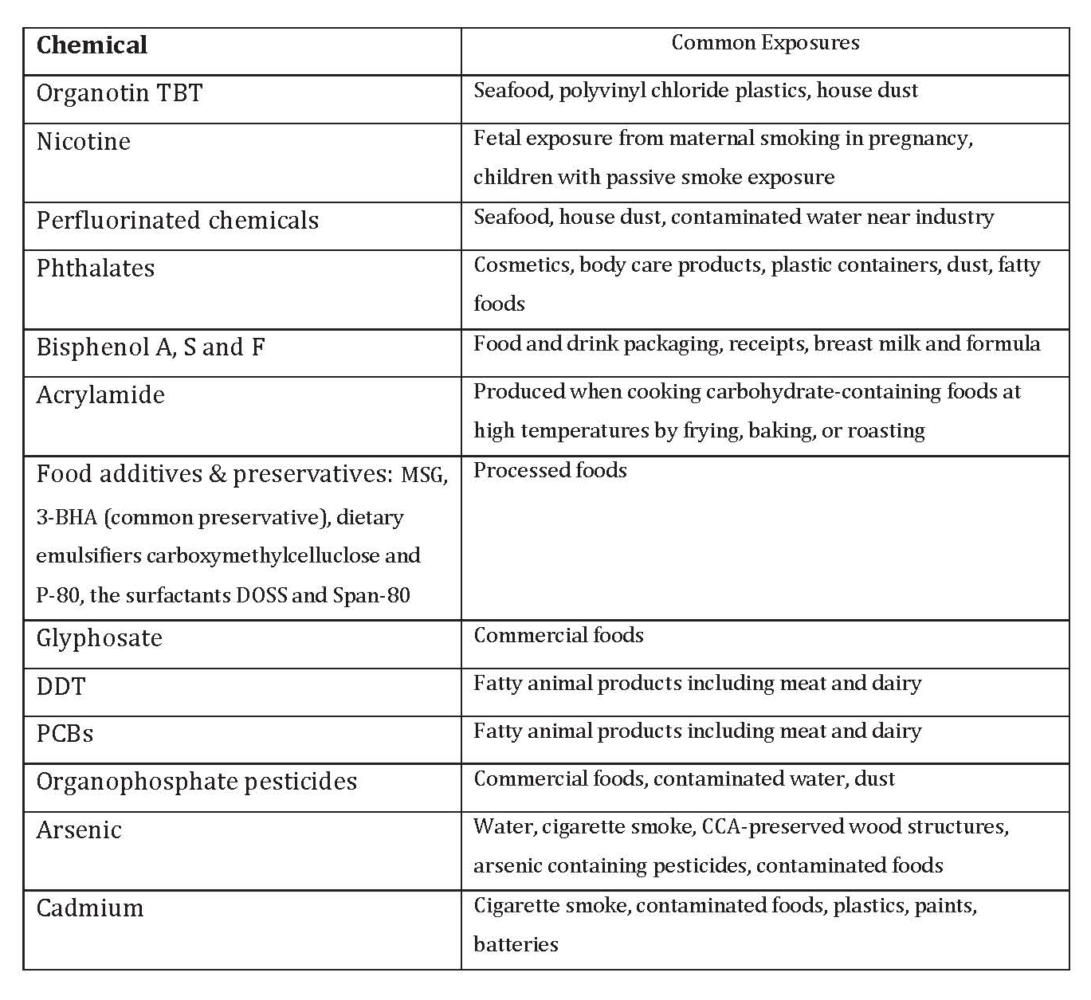
The list of endocrine disruptive chemicals (EDCs) stands at about 1000. Many EDCs have been identified as obesogens and that list is growing steadily as new compounds are added.5 Table 1 offers some examples of classes of chemicals and common exposure routes
Table 1. Endocrine-Disruptive Chemicals
Sleep
Sleep restriction and circadian rhythm disruption are both contributors to altered eating patterns and weight gain. Persistent short sleep patterns, measured as less than seven hours per night, predisposes adolescents and young adults to the development of obesity and increased waist circumference.6 Assessment of the National Health and Nutrition Examination Survey (NHANES), with a sample size of nearly 14,000 adults over the age of 20, demonstrated a linear relationship of increased BMI and waist circumference in those who slept fewer hours. Not only were adults who slept the recommended 7-9 hours more fit, those who slept more than 9 hours had improved anthropomorphic measurements over those sleeping the recommended 7-9 hours per night.7
The suprachiasmatic nucleus (SCN) in the hypothalamus, referred to as the central clock, is the primary mechanism for synchronizing circadian rhythms. The SCN itself is controlled by zeitgebers, environmental and social cues including daylight, artificial light, mealtimes, and timing of exercise/activity. Light-dark cycles have the greatest impact on the SCN, which blocks melatonin production when light is present and stimulates the pineal gland to secrete melatonin when it is dark. Melatonin, in turn, induces sleep, has antioxidant capacity, modulates the SCN, and improves insulin sensitivity. Because all the zeitgebers are controlled by lifestyle choices, this is a promising area of intervention for people with metabolic disease.8
There is an epidemiologic association of diabetes in people who work at night. This phenomenon is hypothesized to occur due to a misalignment between sleep/wake behavioral patterns and internal circadian rhythms, which are governed by daily light/dark sequence and the feeding/fasting cycle. Regardless of when a person sleeps, comparing normal night sleeping to daytime sleep of shift workers, there remains a consistent decrease by as much as 17% in glucose tolerance at 8 PM as compared to 8 AM. This is associated with insulin resistance that is commonly seen in those who work at night.9
Exercise
It has long been recognized that metabolism is modified by physical activity (PA). PA consists of structured exercise, sports, and activities of daily living, including occupation, leisure, and active transport. Metabolic impacts of PA include increased insulin sensitivity and insulin activity, with a reduction of insulin resistance, improved lipid profile, decrease in both fasting blood glucose and hemoglobin-A1C, and weight reduction, specifically visceral adipose tissue.10
While the light/dark cycle is the prime zeitgeber, exercise has been recognized as a secondary zeitgeber that can be used to entrain a health-promoting circadian rhythm. In shift workers and those who frequently change time-zones with travel, exercise has been effectively used to more rapidly re-establish healthy rhythms.11
There is currently significant interest in discovering the best time for exercise. While exercise performance as measured by strength, endurance, and power is more robust in the late afternoon and evening, this does not answer the question of what time of day offers the most optimal health promotion. Studies in mice, muscle cells, and humans confirm this diurnal pattern of enhanced evening performance and illustrate the greater dependance on carbohydrates to produce this exercise efficiency.12 This phenomenon has been successfully utilized by athletes to optimize their performance and set personal athletic records. For metabolically inflexible people, evening exercise may be a successful strategy to lower blood glucose after the evening meal. Conversely, morning exercise may better enhance fat oxidation.
Exercise at 7 AM compared to both 1 PM and 7 PM in 20 prehypertensive men demonstrated improved blood pressure readings. Additionally, deep sleep and overall quality of sleep as measured by the number and duration of nocturnal wakening was significantly better in the early exercisers.13
Timing of exercise in relationship to a meal also has great impact. Pre-meal exercise significantly reduces appetite and food consumption. Research points to exercise-induced suppression of ghrelin, the hormone responsible for increasing appetite and adiposity. Of note, these effects are transient and demonstrate best efficacy when a meal is eaten within 30 minutes of exercise. Pre-meal exercise was also more effective than post-meal exercise at optimizing triglycerides and HDL cholesterol. This effect was longer lasting with effectiveness seen for many hours post exercise. Both pre- and post-meal exercise was effective for prevention of post-prandial hyperglycemia.14
Timing of Meals
While it is evident that energy expenditure must match caloric intake to avoid weight gain, less is said about the effects of the timing of a meal on metabolism. Triglycerides (TG) are higher and remain in the blood stream longer when a high-fat meal is consumed at night as compared to a meal eaten during the day. Comparing lunch to breakfast demonstrates that a mid-day meal produces the lowest blood TG levels whether or not a meal has been eaten at breakfast. This difference is hypothesized to reflect a greater uptake of TG into skeletal muscles cells mid-day. Glucose tolerance on the other hand is best in the morning, and carbohydrates are least tolerated in the evening. Amino acid absorption also shows a diurnal pattern with enhanced morning uptake as compared to the evening. Finally, the mismatch of the sleep/wake and feeding/fasting where calorie consumption is stacked in the evening, leads to metabolic disease and weight gain despite a eucaloric diet eaten that day.15
Not only does a mismatch of circadian rhythms and feeding patterns cause weight gain, but this mismatch is also an obstacle for weight loss and metabolism repair. In a large post-bariatric surgery study, eating the main meal on average 22 minutes later in the day was associated with a greater number of poor responders to this weight-loss therapy despite similar food composition, number of calories, and level of activity.16 In a small study (n=32) of young women on a weight loss program, those who ate lunch at 4:30 PM, as compared to eating at 1:30 PM, showed decreased glucose tolerance and carbohydrate oxidation with a lower resting energy expenditure.17
Fasting
Intermittent fasting (IF) is rapidly becoming a popular and effective tool to address metabolic disorders from obesity to diabetes to cardiovascular disease. Models of IF include a reduced daily feeding window, exemplified in time-restricted feeding (TRF), and alternate day fasting (ADF) with a fasting day followed by ad libitum feeding day. ADF has also been modified to allow one meal on the day of fasting and is referred to as an alternate day modified fast (ADMF). Also popular is the 5:2 diet with 5 days of ad libitum feeding and 2 days of fasting, which can be back-to-back or separated by feeding days. When compared to daily caloric restriction (CR), these diets perform equally well for weight loss, diet adherence, and for optimizing most cardiometabolic markers. However, fasting models consistently outperform CR for reducing insulin resistance.18 For this reason, IF offers more promise for reversing metabolic inflexibility while weight loss goals are being met.
The effectiveness of intermittent fasting for weight loss is frequently attributed to an overall reduction in calories similar to CR diets. In a study comparing ADF with ad libitum 8-hour TRF, ADF demonstrated 4-6% weight loss in 12 weeks vs. roughly 3% in 12 weeks for TRF. This effect was hypothesized to be due to the overall greater caloric restriction of ADF.19
To date, IF and CR studies show inconsistent and conflicting results in terms of cardiometabolic improvements beyond the effects of IF on insulin. This may be due to additional factors such as exercise, sleep, stress, cultural norms and behaviors, macronutrient balance, and time of eating related to circadian rhythms.20 Furthermore, food quality, optimal nutrient support, toxicant exposure, and genetic differences are likely to impact metabolic pathways driving metabolic disease. Finally, digestive health imbalances and microbiota dysbiosis are associated with and have been shown to drive metabolic illness. This topic alone requires an in-depth understanding beyond the scope of this article.
Where Modern Life “Messed It Up” and How to Get Back on Track
In industrial countries where MetS is prevalent, most people are exposed to calorie-dense nutrient-poor food on a daily basis, while rarely facing food shortage. This shifts metabolism toward fuel storage that is not needed at a future date. This one-way path leads to macronutrient excess in the blood stream as well as ever increasing fat stores. Metabolically, this is driven by inappropriate insulin activity and response.
It has long been recognized that the lifestyle modification of diet, exercise, and circadian rhythms is key to reversing metabolically driven chronic disease. While blood sugar dysregulation, excess weight, and obesity are common features of metabolic illness, caloric restriction and weight loss alone often do not heal the underlying disorder. This has been demonstrated repeatedly both clinically and scientifically. Cardiometabolic profiles do improve with weight loss achieved through caloric restriction; however the metabolically dysregulated profile returns as soon as a eucaloric diet is resumed. To achieve lasting health benefits, metabolism must return to the flexible state.
There is considerable disagreement regarding the best time for eating, sleeping, and exercise to achieve positive and permanent results. Likewise, there are conflicting arguments pitting a low-fat diet against a low-carb diet. Clinically there is always a balance between what science demonstrates and what the patient in front of the clinician exhibits.
This is where the art of medicine dictates intelligent experimentation and individualization. To heal metabolism, it is important to address an individual’s cultural norms, personal preferences, and work requirements. The next step is to introduce a nutrient-dense minimally processed and low-pesticide diet. This is followed by sleep optimization and a manageable physical activity schedule. Once these measures are instated, assess and minimize toxicant exposure of obesogens and consider reducing endogenous stores of these compounds. The plan created for this individual is then assessed and adapted on a regular schedule over a period of months to years. Once metabolic flexibility is reinstated, a supported plan to maintain MetF is often essential to create lasting health.
References:
- Zhang, S., et al.The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond) 11, 10 (2014).
- Smith RL, et al. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr Rev. 2018;39(4):489-517.
- Freese J, et al. The sedentary (r)evolution: Have we lost our metabolic flexibility?. F1000Res. 2017;6:1787. Published 2017 Oct 2.
- Egusquiza RJ, Blumberg B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology. 2020;161(3):bqaa024.
- Darbre PD. Endocrine Disruptors and Obesity. Curr Obes Rep. 2017;6(1):18-27.
- Krueger PM, et al. Cumulative exposure to short sleep and body mass outcomes: a prospective study. J Sleep Res. 2015 Dec;24(6):629-38.
- Ford ES, et al. Sleep duration and body mass index and waist circumference among U.S. adults. Obesity(Silver Spring). 2014 Feb;22(2):598-607.
- Quante M, et al. Zeitgebers and their association with rest-activity patterns. Chronobiol Int. 2019 Feb;36(2):203-213.
- Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225-E2234.
- Wake AD. Antidiabetic Effects of Physical Activity: How It Helps to Control Type 2 Diabetes. Diabetes Metab Syndr Obes. 2020;13:2909-2923.
- Gabriel BM, Zierath JR. Circadian rhythms and exercise – re-setting the clock in metabolic disease. Nat Rev Endocrinol. 2019 Apr;15(4):197-206.
- Ezagouri S, et al. Physiological and Molecular Dissection of Daily Variance in Exercise Capacity. Cell Metab. 2019 Jul 2;30(1):78-91.e4.
- Fairbrother K, et al. Effects of exercise timing on sleep architecture and nocturnal blood pressure in prehypertensives. Vasc Health Risk Manag. 2014 Dec 12;10:691-8
- Reid RER, Thivel D, Mathieu ME. Understanding the potential contribution of a third “T” to FITT exercise prescription: the case of timing in exercise for obesity and cardiometabolic management in children. Appl Physiol Nutr Metab. 2019 Aug;44(8):911-914.
- Aoyama S, Shibata S. Time-of-Day-Dependent Physiological Responses to Meal and Exercise. Front Nutr. 2020 Feb 28;7:18.
- Garaulet M, et al. Timing of food intake predicts weight loss effectiveness [published correction appears in Int J Obes (Lond). 2013 Apr;37(4):624]. Int J Obes (Lond). 2013;37(4):604-611.
- Lopez-Minguez J, Gómez-Abellán P, Garaulet M. Timing of Breakfast, Lunch, and Dinner. Effects on Obesity and Metabolic Risk. Nutrients. 2019 Nov 1;11(11):2624.
- Hoddy KK, et al. (2020), Intermittent Fasting and Metabolic Health: From Religious Fast to Time‐Restricted Feeding. Obesity, 28: S29-S37.
- Gabel K, et al. Effects of 8-hour Time Restricted Feeding on Body Weight and Metabolic Disease Risk Factors in Obese Adults: A Pilot Study. Nutr Healthy Aging. 1 June. 2018: 345 – 353.
- Rynders CA, et al. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients. 2019;11(10):2442.
Author bio:
During her 20 years of practice, Dr. Nedrow has worked in midwifery, women’s health, family medicine, and healthy aging. She has had the opportunity to support nearly a thousand patients through her cleansing and metabolic healing programs. Dr. Nedrow is the President of the National Association of Environmental Medicine (NAEM) and a widely recognized speaker on topics that include naturopathic medicine, environmental medicine, detoxification, and keto-adaptation. She is the author of three books, including the most recently published, Metabolic Flexibility, How to Heal Your Metabolism with a Ketogenic Diet.
Consult your doctor before using any of the treatments mentioned in this article.
Reprinted with permission from the May 2021 Townsend Letter, and Bonnie Nedrow, ND.
Learn how you can benefit from more Townsend Letter articles.