Article from Townsend Letter
DHEA Physiology, Deficiency, and Treatment
by Alan B. McDaniel, MD
Introduction: “You never forget your first.”
Case 1: In the early 1990s, a slender, older man asked Doc to help him regain some energy. He had been fatigued for nine years, ever since operative treatment for base of tongue cancer followed by external beam radiotherapy. On examination, his palms were not anemic, but his facial capillaries were so under-perfused that he was the same color as Doc’s manilla-folder chart.
Doc ordered a lab workup, recommended nutritional supplements (including desiccated neonatal adrenal cortex) and having heard it might support adrenal function, made him the first patient he ever treated with DHEA. Six weeks later, on returning to review his laboratory results, the patient virtually radiated energy and enthusiasm. His cheeks were ruddy, and he said he had not felt so well in fifteen years. He thanked Doc and then never returned! What is DHEA and why did it help this man so much?
The Scientific Basis of DHEA Therapy
What is DHEA? Made from cholesterol, dehydroepiandrosterone (3β-hydroxy-5-androsten-17-one, DHEA; Figure 1) and its sulfated metabolite DHEA-S (Figure 2) are the major steroid secretory products of the human adrenal glands.1 DHEA is also produced in the gonads, brain, and gastrointestinal tract.2 DHEA and DHEA-S are the most abundant circulating steroids, present in quantities second only to cholesterol.3 Dehydroepiandrosterone is a precursor for both androgenic and estrogenic steroids and of neurosteroids.1
How DHEA is produced and processed? All steroid hormones are derived from cholesterol. The first step occurs in mitochondria, when cholesterol is converted to pregnenolone. This is regulated in the adrenals by adrenal corticotropic hormone (ACTH); luteinizing hormone (LH) in the gonads and in other mitochondria by an independent side-chain cleavage enzyme.4
The steroidogenic enzymes and pathway for DHEA synthesis in the adrenal gland are reviewed by Miller and Auchus, 2011.5 In the adrenal cortex’s zona reticularis, pregnenolone is converted in two steps to DHEA by the enzymes 17α-hydroxylase and then17,20-lyase (the latter is regulated by LH).2
At this point, DHEA diverges onto two paths, which later rejoin: The enzyme 3ß-HSD takes DHEA “south” to produce androstenedione (of Mark McGwire fame). The enzyme 17ß-HSD (of which there are five isoforms) moves DHEA “east” to become androstenediol.6 Then, in a “onesie-twosie,” each enzyme will then move the other product of DHEA respectively “east” and “south” and both pathways end at testosterone (Figure 1.).
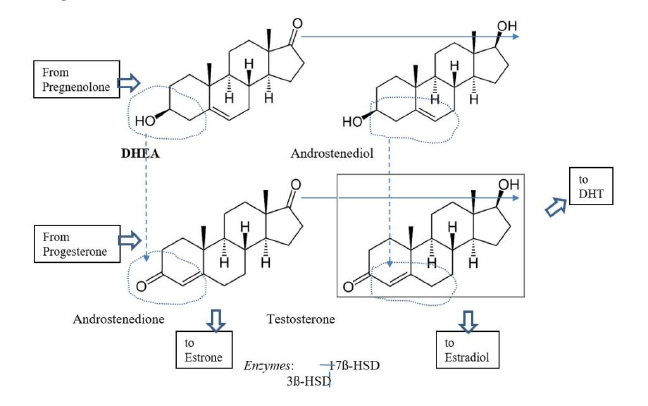
Figure 1: Metabolism of DHEA to testosterone
DHEA production is regulated by LH, which controls 17-20 lyase activity. Testosterone production is governed by the rate-limiting action of 17β-HSD. Its many isoforms are controlled by various agents relevant for specific tissue type and function – in the testes, it is largely by FSH. Activity of the enzyme 3β-HSD is less strictly governed but can be enhanced when needed and is down-regulated as downstream products accumulate.7
Testosterone is a “reasonably potent androgen” but it also should be considered a precursor.8 Testosterone is the parent of both the strongest feminizing hormone, estradiol, via the enzyme aromatase and of the most potent androgen, dihydrotestosterone (DHT), through 5alpha-reductase.9,10
DHEA is both a precursor and a (weak) hormone. In the classical sense, DHEA is a hormone precursor. It is converted to testosterone, then to either estradiol or DHT, both in gonads and in various other tissues.3 DHEA (but not DHEA-S) binds with weaker affinity to various nuclear receptors (NRs), including androgen-receptor, estrogen-receptors (both alpha and beta), PPAR and others.11 In addition to directly binding to NRs, DHEA has been shown to modulate the levels of NRs.2 “DHEA actions are classically associated with age-related changes in cardiovascular tissues, female fertility, metabolism, and neuronal/CNS functions” 2,12 and this list is incomplete.
Additionally, DHEA intrinsically has potent biological effects via binding to non-genomic receptors on cell-surfaces.13 It also acts on various neuroreceptors, including GABA, NMDA and sigma-1 receptors.14 The consequences include activating various membrane receptors and inhibiting voltage-gated T-type Ca2+ channels.2
The neurosteroid effect of DHEA is of particular interest. It and DHEA-S easily enter the brain across the blood-brain barrier. The brain itself also locally synthesizes them, so their concentrations in the CNS are particularly high.15 The CNS actions of DHEA and DHEA-S include neuroprotection, neurite growth, and antagonistic effects on oxidants and glucocorticoid activity.16 Most effects of these neurosteroids (or “non-classical steroids”) are non-genomic, influencing immune reactions, mood and emotions, and behavior.17 Therefore, it is observed: “DHEA has considerable effects on mood, well-being and sexuality in patients with adrenal insufficiency, and also in those with mood disorders.”18
The immune-modulatory effect of DHEA is reported in the elderly, in whom it increases the number of monocytes, T cells expressing T-cell receptor gamma/delta (TCRγδ) and natural killer (NK) cells. It also can improve their physical and psychological well-being, muscle strength and bone density. DHEA reduces body fat and age-related skin atrophy by stimulating procollagen and sebum production.19
The relationship of DHEA and DHEA-S. In the adrenal cortex, DHEA is conjugated with a sulfate ester to form DHEA-S (Figure 2). Such conjugation hinders it – as it does many other steroids – from binding to nuclear receptors and acting on the genome.13 However, DHEA-S retains non-genomic neurosteroid-activity.20 In years past, DHEA and DHEA-S were thought to be rapidly inter-converted; this has been revised: While DHEA-S can be processed into DHEA and sex hormones in the peripheral tissues (by “intracrinology”),21 DHEA-S is largely a “one-way conjugation product,” not a significant hormone precursor.4 Significantly, oral DHEA doses are extensively sulfated in the hepatic first pass.22 Clinically, it is prudent to consider DHEA and DHEA-S as similar but separate actors.
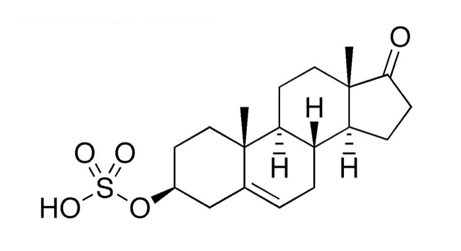
Figure 2. DHEA-Sulfate
https://en.wikipedia.org/wiki/Dehydroepiandrosterone_sulfate
Circulating DHEA and DHEA-S are not maintained in equal balance, as once was believed. Sex hormone binding globulin (SHBG) has high affinity for DHEA-S but only weakly binds DHEA. Thus, DHEA is largely free and highly metabolically active, while DHEA-S (being carried by protein) is much the most abundant circulating steroid, after cholesterol.23
DHEA in maturation and aging. After briefly robust levels decline shortly after birth, circulating adrenal antigens remain quite modest in childhood. The production of DHEA, DHEA-S and androstenedione again increases around age 8 or 9, as the zona reticularis matures in an event called adrenarche.24-27 These effects remain subtle for a few years: Most DHEA is inactivated by conjugation to DHEA-S until near-puberty.
Following puberty, serum DHEA levels rise and peak between age 20 and 30 years. The majority of it is made in the adrenals, with about 15% arising from the gonads.28 Serum DHEA and DHEAS levels thereafter drop by 2% every year, due to attrition of adrenal cortical and (in men) of Leydig cells. In women at menopause, only 60% of the former peak values are present.29 At age 70, amounts are just 20-23% of what they had once been and by age 80, can fall to as little as 10% of the former supply.30
Serum androgens were tested yearly in women ages 45 to 54 and no independent effect of menopause was noted.31 After menopause, the ovaries contribute a significant amount of DHEA (up to 50%) even ten years later.32 Postmenopausal oophorectomy causes a significant drop in androgens, including testosterone.31 Labrie states that intracrine metabolism of DHEA to sex hormones supplies menopausal women’s tissues with essential sex steroids – and that low DHEA leads to a deficiency of these hormones.33
Conditions Associated with Low DHEA
Stress: The acute stress response is associated with a transient (one-hour) increase of circulating DHEA,34 which arises from the adrenal cortex.35 Women, young adults, and obese individuals produced the greatest levels.36 DHEA will be increased following acute mental stress, whatever the type or duration. Overall, DHEA is increased in the successful response to stress.37
When the stressful event is prolonged, hypothalamic release of ACTH is enhanced and cortisol rises. Simultaneously, LH and FSH are inhibited, and androgens fall.38 Following a significant injury, sixty patients lost testosterone, DHEA, and DHEA-S.39 Importantly, plasma androgen levels correlated with the Sequential Organ Failure Assessment (SOFA) scores and sepsis. Muscle wasting peaked at six weeks. Normal amounts of testosterone were restored after two months, DHEA in four months and DHEA-S after more than six months. This may indicate a “hierarchy of need” for a depleted but recovering resource, DHEA.
In most cases, the chronic stress response features continued up-regulation of the hypothalamic-pituitary-adrenal (HP-A) axis and the ongoing inhibition of sex hormones.40 While the concept of “adrenal fatigue” (academicians always use the quotation marks!) is controversial, adrenal reserve capacity can be depleted and thus, DHEA production would be also.34,41
It is observed that “HPA axis dysregulation has been found in a high proportion of chronic fatigue syndrome (CFS) patients.”42 Investigations show these CFS and chronic inflammation patients have low DHEA values.43,44 However, a later meta-analysis of 17 studies of post-traumatic stress disorder patients found no significant differences from control groups in either DHEA or DHEA-S.45
Toxins: Endocrine disrupting chemicals alter androgens.46 Prenatal xenobiotics cause fetal HP-A axis dysfunction, which may affect the brain’s neurosteroids, neurotransmitter function, cognitive ability, and neural immune-regulation in the child.47
Aging: A substantial decline of serum DHEA values in men after age 20 – 30 was mentioned above.48 In what way is this physiological decline related to the process of aging? It has been persuasively demonstrated that men with lower testosterone levels have a worse state of health49,50 and that lower levels of testosterone may be a risk factor for frailty in aging men.51,52
A similar association is reported for lower circulating DHEA levels and frailty.53,54 A greater decline in circulating levels of androgen over five years is associated with increased all-cause and cause-specific mortality in older men.55 Indeed, falling DHEA and DHEA-S levels are associated with many age-related disorders, including metabolic and cardiovascular diseases, poor physical performance, mood and memory defects, poor sense of wellbeing, and sexual dysfunction.56,57
Women also suffer from age-related declining DHEA. As above, Labrie believes low DHEA is important, as intracrine activation of DHEA to sex steroids protects menopausal women. In a study of post-menopausal women aged 50 to 90 years, age-adjusted loss of DHEA was significantly associated with sarcopenia.58
Depression is associated with lower DHEA values.59-61 A neurosteroid effect seems likely, as NHANES data shows serum testosterone values are inversely associated with cognitive performance in older men in the US (but not in women).62
Anorexia nervosa: Decreased secretion of DHEA in this condition is accompanied by other endocrine abnormalities due to malnutrition and chronic stress. Along with high cortisol, the low levels of IGF-1, estrogen, testosterone, and DHEA are associated with “profound bone mineralization disorders.”63
Elevated DHEA in women: The overproduction of adrenal androgens occurs in nonclassical 21-hydroxylase-deficient congenital adrenal hyperplasia.57 Androgens are also elevated in polycystic ovarian syndrome, although perhaps less of adrenal origin than due to the dose-related effects of insulin on the ovary.64,65 Acanthosis nigricans is associated with elevated DHEA-S due to insulin resistance.67
Controversy: Is DHEA Supplementation Beneficial?
The rationale is simple: DHEA production declines significantly with age, stress, and illness. The levels of steroid hormones derived from DHEA also fall. These reductions (or deficiencies) impair normal function and are significantly associated with many degenerative conditions, as well as earlier mortality. Replacement may help: “Epidemiological evidence from humans and animal studies suggest that DHEA/DHEA-S may have cardioprotective, anti-obesity, antidiabetic, and immuno-enhancing properties.”67
Supplementing DHEA should replenish its loss, making the precursor available for glandular and intracrine conversion56 and for its neurosteroid effects. Furthermore, this supplement bypasses 17-20 lyase, the enzyme that is “inactivated” when LH is inhibited by chronic stress. As proof of this concept, DHEA supplements yield twice as much testosterone as will equally dosed precursors “upstream” of 17-20 desmolase: Pregnenolone, progesterone &17α-OH progesterone.68 Adding DHEA “distal” to 17-20 lyase also raises progesterone levels (and probably the other listed precursors),69 presumably because less are converted to DHEA. Patients’ responses indicate this increases the synthesis of cortisol and aldosterone (as needed).
DHEA is sold over-the-counter in the US; and in sensible hands, the use of a precursor instead of an active hormone is quite safe.56 Because “downstream” enzymes are regulated (by feedback from their products, by FSH and probably more), the provision of excessive precursor will not force the production of unwarranted hormones.70 Reliable proof of this safety is found in studies of men with normal testosterone given labeled DHEA supplements; they did not make any excess of testosterone, though some exogenous DHEA had been transformed into testosterone.71-74 The undesirable effects of injudicious dosing (largely intracrine) are reviewed in “Side Effects.”
Case 2 is a 32-year-old police officer whose chief complaint in 2020 was “I just feel like I’m tired.” His mother is hypothyroid and his family history is positive for diabetes and other conditions related to insulin resistance. He feels stressed, is irritable and easily loses his temper. He has been treated for allergies since childhood.
His baseline laboratory evaluation showed: total testosteroneLCMS= 211.6 L (264-916 ng/dL); free testosteroneED= 5.5 (5.0-21.0 ng/dL) and total estradiolLCMS= 17 (8-35 pg/mL) with calculated tTest/E2 ratio=12.4 (units not corrected). After treatment with desiccated adrenal cortex and liothyronine (T3) to correct dysfunctional deiodination75 (ratio of tT3/ RT3= 5.7 was raised to 13.0), he began taking DHEA 25 mg daily at bedtime.
On these treatments and a somewhat healthier diet, his next lab values showed: total testosteroneLCMS= 466.2; free testosteroneED= 14.56 and total estradiolLCMS= 21. His calculated tTest/ E2 ratio= 22.2 (units not reconciled), which is much more to his doctor’s liking.
Evidence That Treatment with DHEA Is Beneficial
Although this caution seems hardly necessary in an article for practitioners, let it be recognized: “It is important to remember that numerous medical conditions and medications can result in low androgen levels in women.”76That’s true for men, too. Now, assuming patients have been appropriately evaluated, let us consider treatment with DHEA and androgens.
Testosterone: DHEA can restore testosterone and androgenic effects to patients with Addison’s disease. Many studies have proven this, particularly in women, for whom the adrenal glands had been the major source of DHEA.73,77,78 Beyond Addison’s, a recent meta-analysis of 42 RCTs demonstrated that testosterone level was significantly increased after DHEA administration.79 Other particular situations in which testosterone levels rise with DHEA supplementation include hypogonadal men80-82 and soldiers stressed during survival training.83
Male sexual function: Despite the encouraging testosterone gains from DHEA, publications studying male sexual function find inconsistent results. Some studies report positive effects on male sexual function.84-86 Others discover no effects at all for men.87,88 Beyond the essential role of nitric oxide in erections, it may be that the endocrinology of libido is not completely understood. It is also noteworthy that no man got worse on DHEA.
Women’s sexual function: The “global consensus position statement” on testosterone for women’s sexual function, based on the available evidence from placebo/comparator randomized controlled trials (RCTs), is positive.89 It states: “Testosterone therapy, in doses that approximate physiological testosterone concentrations for premenopausal women (original emphasis), exerts a beneficial effect on sexual function.” This includes many domains, including sexual desire, arousal, orgasmic function, pleasure, responsiveness and more (Level I, Grade A). The statement reverses older, negative position-papers.90,91
This is not proof that DHEA will be successful. However, we have seen that DHEA supplementation can significantly increase women’s testosterone.79 It is also unlikely that DHEA could produce supra-physiological levels of testosterone, against which italicized emphasis in the recent global position statement warned us. Still, authors from the Mayo Clinic and Cleveland Clinic caution us: “DHEA currently is not approved to treat sexual dysfunction.”76 It seems fortunate that DHEA is OTC-available in the US and that the only required approval is the patient’s informed consent. When recommending it to women, follow their therapeutic levels and bear in mind: A good clinical response is often realized but it is not a certainty.
Women’s fertility: Meta-analysis of nine studies involving 540 women pre-treated with DHEA for diminished ovarian reserve (DOR) found clinical pregnancy rates were significantly increased (OR=1.47).92 Women with DOR who received 75 mg of DHEA daily had increased baseline follicular phase progesterone and no adverse effects on the cycle outcome.69 Beyond its classical role as sex hormone precursor, DHEA (and DHEA-S) may be “oocyte factors,” through their nongenomic effects on calcium channels.93
Among 22 in-vitro fertilization patients, pre-treatment with DHEA improved many measures significantly over the control group, including anti-Mullerian hormone, antral follicle count, serum estradiol, the number of recovered oocytes, fertilized oocytes, the fertilization rate and most importantly, the live birth rate (P< 0.05).94 The usual dose of DHEA employed is a robust 75 mg daily, which reportedly has “no serious side effects.”95 Unfortunately, the more severe “premature ovarian insufficiency” has not responded to DHEA supplementation.
Menopause: Having earlier noted that androgens fall but little after menopause, it should be no surprise that aromatase and 17β-HSD alterations, not DHEA deficiency, explain the great postmenopausal decline in estradiol. Even in large doses, DHEA cannot restore follicular blood levels of estradiol in menopausal women.96
While a meta-analysis of randomized controlled trials (RCTs) involving 1,233 women found DHEA 50mg/day supplementation increased estradiol significantly, the gain was small (weighted mean 7.02 pg/mL and aged ≥60 years, WMD: 8.56 pg/mL).97 Unfortunately, this study does not report the resulting testosterone gain or its ratio to estradiol, that one could compare the relative changes in these counter-balancing hormones. The author’s experience suggests testosterone would be increased relative to estradiol.
While these estradiol gains are very small, Labrie, the Mahatma of Intracrinology, suggests they are sufficient. He states locally made, sex steroids exert their action and are then inactivated intracellularly without significant release into the systemic circulation. Thus, although DHEA supplements hardly increase estradiol blood levels, sufficient tissue effect would be received due to intracellular aromatization.98
In the author’s practice, most menopausal women benefit from adding estradiol and progesterone (or pregnenolone) to DHEA. It seems desirable to keep all steroid sex hormones in a physiological balance normal for young women. This statement is validated by patient care and research: “…A reasonable dose of DHEA given to perimenopausal women demonstrated that normal testosterone levels may be achieved consistently in most if not all of many published reports.”96,99 Noting that testosterone replacement therapy in women is generally discouraged (except for sexual function),90,100 the success of OTC DHEA is reassuring.
Osteopenia: Until lately, review articles had claimed “no evidence” to support testosterone therapy for women’s bone health.101,102 Two recent meta-analyses of RCTs103,104 reverse this and show DHEA supplementation increases women’s bone density, as it does their serum testosterone. An intracrine effect contributes to bone density, as androgens are converted to estradiol in the bone.105
Vaginal atrophy: A position statement of The North American Menopause Society on the management of the genitourinary syndrome of menopause (GSM) concluded: Vaginal DHEA is effective treatment for moderate to severe GSM.106 The human vaginal smooth muscle cells synthesize “downstream” androgens from DHEA.107 Vaginal DHEA (prasterone) has been approved to treat moderate to severe dyspareunia.76 Compared to placebo and moisturizers, intravaginal DHEA (prasterone) 6.5 mg for 12 weeks improves vaginal pH, cell maturation, dyspareunia and significantly improved sexual health and related domains (P < .0001).108-110
Intravaginal DHEA has no adverse effects on the endometrium after 12 months of therapy.111 Most studies suggest no significant increase in serum levels of steroid sex hormones with the use of vaginal DHEA.112 Breast cancer survivors were studied. While intravaginal DHEA increased circulating DHEA-S, testosterone, and estradiol values, they remained within the normal postmenopausal ranges.112 Authors of a recent review cautiously stated: “Women who have no history of estrogen-dependent cancers should be routinely offered treatment for GSM with vaginal estrogen or DHEA.”76
Breast Cancer: There is neither consistent data nor a substantial association of either DHEA or DHEA-S to women’s risk of breast cancer.113-115 It is hypothesized that the effects of androgens depend on concurrent estrogen levels. In-vitro, DHEA-S (but perhaps not DHEA) can compete with estradiol for ERα and ERβ-binding.116,117 Thus, before menopause, DHEA/S will exhibit anti-estrogenic effects but after menopause it becomes weakly estrogenic. DHEA was also found to inhibit the proliferation and migration of tumor cells derived from the breast.118
Supplementation should not be to an excess, though: Elevated levels of androgens (free testosterone, DHEA-S and androstenedione) have been correlated with an increased post-menopausal breast cancer risk.119 It is believed the activated androgen receptor can stimulate cellular proliferation of estrogen receptor-negative breast cancer.
Fibrocystic breast disease: Androgens may protect women from fibrocystic breast disease.120
Primary hypoadrenalism (Addison’s): As above, many reports validate the benefit of DHEA supplementation in Addison’s disease, from replenishment of androgens. In addition to restoring circulating DHEA, DHEA-S and androstenedione levels to these people, DHEA supplementation reduces total cholesterol, improves well-being, sexual satisfaction, and insulin sensitivity; and it prevents the loss of bone mineral density.19
Adrenal “fatigue”: The chronic fatigue syndrome (CFS) shares at least 36 features with Addison’s disease.121 Whilst Cleare et al. have shown low-dose hydrocortisone successfully improves the symptoms of CFS,122,123 he does not recommend it as a chronic therapy, for it suppresses the already dysfunctional HPA-axis and further depresses steroid sex hormones.124 DHEA has been evaluated as an alternative to hydrocortisone.
Some studies report low DHEA in chronic fatigue syndrome patients.59 As above, adding DHEA “distal” to 17-20 lyase increases the quantities of progesterone and other precursors of aldosterone and cortisol.69 Thus, DHEA might help relieve adrenal symptoms without the drawbacks of using hydrocortisone. This outcome was reported in a small study.125
Depression: Neurosteroids significantly affect mood and DHEA-S enhances glutamatergic signaling. A meta-analysis of RCTs found a significant effect in favor of DHEA treatment for depression compared to placebo.126 The authors concluded that DHEA may be an additional, effective alternative to antidepressants.
Insulin resistance – Cortisol: The metabolic syndrome is associated with functional hypercortisolemia.127 A meta-analysis confirmed that DHEA has a cortisol-blocking effect: It found supplementation with DHEA significantly decreased cortisol levels and there were no differences in adiponectin, leptin, or liver transaminases. The authors concluded DHEA may be used to treat hypercortisolemia and it is safe for the liver.128
Insulin resistance – Obesity: A meta-analysis of 25 placebo-controlled trials of DHEA supplementation in elderly men (n= 1,353) with 36 weeks mean-follow-up confirmed a reduction of body fat-mass.129 A more recent meta-analysis of 23 RCTs also found DHEA supplementation decreases fat mass and increases lean body mass.130 Some of this benefit may be due to the ability of DHEA and its metabolite androstenediol to activate nuclear peroxisome proliferator-activated receptors (PPARs)—transcription factors, which are involved in regulating the body’s lipid homeostasis.131
Insulin resistance – Lipids: DHEA activates PPARs. Gemfibrozil activates PPARα; pioglitazone selectively stimulates the gamma (PPARγ) and to a lesser extent, PPARα. Therefore, one would expect DHEA to have a beneficial effect on lipid metabolism and blood lipids, of which there is “much experimental evidence.” 132 However, a recent meta-analysis of RCTs failed to substantiate this.133 They reported no significant difference in total cholesterol, LDL-cholesterol, and triglycerides but rather a statistically significant (but clinically insignificant) decrease in women’s HDL-cholesterol (not men’s).
Insulin resistance – Diabetes: Blood levels of DHEA are inversely correlated with the risk of type-2 diabetes,134 which might be coincidental. Importantly, studies reported that DHEA replacement improved insulin sensitivity,135,136 and glucose tolerance (GT) but only in participants with initially abnormal GT. It also reduced plasma triglycerides, and the inflammatory cytokines IL6 and TNFα.137 While a meta-analysis revealed that once diabetes is established, DHEA supplementation improves the fasting glucose by a statistically significant number, it was by only a tiny 2.2 mg/dL.138
Cancer: DHEA protects against several types of cancer,139 particularly cervical cancer. DHEA inhibits proliferation and induces the death of cervical cancer cells (regardless of HPV status) by mechanism(s) independent of androgen and estrogen-receptors.140 This anti-proliferation effect on cervical cancer cells was associated with decreased cellular adhesion and migration. These findings, along with reduced angiogenesis and capillary tube formation were earlier described in-vitro, at high concentrations of DHEA.141
Inflammation and immunity: Here again, authors’ cautions may temper our enthusiasm over the following information: “Much of the research is conducted on rodent models using very high concentrations of hormone supplements, which may not meaningfully translate to human physiology. The convoluted nature of DHEA-immune interactions makes direct effects difficult to interpret.”142
DHEA has differential actions on human immune function and its effects are shaped by the concurrent concentrations of other hormones. DHEA is generally considered immune-supportive but has also been shown to inhibit certain facets of innate and cell-mediated immunity, suggesting complex roles.143 DHEA has an anti-glucocorticoid effect.142 In asthmatic airways, DHEA/DHEA-S attenuate T helper-2 allergic inflammation and reduce eosinophilia and hyperreactivity.19 DHEA specifically inhibits interleukin-6, which could be useful for COVID-19 infections.144
A 2014 review catalogued beneficial effects of DHEA for inflammatory processes, both demonstrated and hypothesized.19 DHEA exerts an anti-inflammatory, vasorelaxant, and anti-remodeling effect to modulate cardiovascular signaling pathways. It is consistent that its low levels correlate with increased cardiovascular disease and all-cause mortality. DHEA/DHEA-S appear protective in asthma and allergy. In an unblinded study, it induced remission in most patients with inflammatory bowel disease. In systemic lupus erythematosus, DHEA is steroid-sparing. Its use for rheumatoid arthritis has been proposed.
Growth hormone: Meta-analysis of 24 trials found that serum IGF-1 increased significantly with DHEA treatment compared to controls.145 Subgroups showed significantly increased IGF-1 in women (but not men) aged >60 years without underlying co-morbidity, who took 50 mg DHEA daily for at least 12 weeks.
Cognition: A systematic review of RCTs studying the effects of DHEA supplementation on cognitive functioning in non-demented men of middle-age and older reports the existing evidence is scanty and “severely inconsistent.”146 Despite the known neurosteroid and protective effects, the authors found no evidence of any benefit.
Aging: Chrousos et al. state regarding anti-aging use: “DHEA supplements do not comply with evidence-based medicine.”56 The AMA House of Delegates resolved in 2009 that “The use of hGH and DHEA as anti-aging agents is not recommended.”147
The clinical response of selected patients to DHEA supplementation would contradict these statements (Case 1). No published trial has joined the nutrients provided in desiccated adrenal glandular with DHEA treatment, as described below in Case 3. An automobile will not function well, regardless of the amount of gasoline in the tank if there is no oil in the engine.
The position statement of the Polish Menopause and Andropause Society is current and sensible.148 They write: “DHEA supplementation is effective in women with adrenal insufficiency and chronically treated with exogenous glucocorticoids, postmenopausal women with low bone mineral density and/or osteoporosis, premenopausal women with sexual disorders and low libido, and in women with vulvovaginal atrophy due to menopause or genitourinary syndrome of menopause.”
They continue: “Currently available clinical trials also suggest that DHEA supplementation is probably effective in postmenopausal women with hypoactive sexual disorders, infertile women with diminished ovarian reserve, women suffering from depression and anxiety, and women with obesity and insulin resistance. No serious adverse effects have been reported.” It is pleasant that they do not forbid using DHEA in any particular circumstance due solely to “lack of evidence.”
Successful DHEA Therapy
Goals and rationale: The goal of DHEA supplementation is to improve deficient patients’ health by restoring their hormone balance at optimal levels. We hope to normalize adrenal function, to restore the steroid sex hormones to physiological levels of younger adults, and to facilitate optimal neurosteroids so they may reap the many benefits catalogued above.
Biochemical bottlenecks exist at multiple levels, which can prevent DHEA synthesis. In both acute and chronic stress, depressed gonadotropin secretion (LH and FSH) reduces the actions of 17-20 desmolase and 17β-HSD.38 If ACTH secretion is impaired, the adrenal produces less pregnenolone, the universal steroid precursor from which steroid hormones are made. Supplying the body with DHEA and pregnenolone gives the adrenal glands access to supplementary precursors.149 These precursors provide the body with partly assembled pre-hormones that either can be processed to hormones or expelled as waste products.
Supplementing DHEA bypasses the step impaired by the loss of LH and replenishes the pathways to testosterone and its products, estradiol and DHT.150 As evidence, we see that giving DHEA yields twice as much testosterone as can equally dosed precursors “upstream” of 17-20 desmolase: Pregnenolone, progesterone, and 17α-OH progesterone.68
Safety: Enzymes that produce “downstream” steroids are regulated. Therefore, precursors cannot force the excessive production of hormones.70 People given even large doses of DHEA will not make too much cortisol.96 It has repeatedly been demonstrated that no amount of DHEA supplementation will cause a man with normal testosterone to make more than a normal amount of it.71-74
Excessive amounts of DHEA are generally conjugated to DHEA-S and cleared.71,151 On 24 hour-urine adrenal steroid profile, elevated androstenedione “waste” products (androsterone and etiocholanolone) also indicate an overly large DHEA dose. Do not take this to mean that correct dosing is irrelevant! Signs and symptoms of androgen excess (e.g., acne of the face and back, hair loss and hirsutism) can follow a large dose due to its intracrine activation in the skin, which is a capable steroidogenic organ.152 This is particularly true for women with polycystic ovary syndrome, for whom a “normal” dose of DHEA is often too much.
Diagnose low-DHEA. Patients with adrenal dysfunction should be tested for DHEA at some time in their therapeutic course. The 2016 Endocrine Society clinical guidelines for diagnosing adrenal dysfunction are freely accessible online.153 As noted above, exogenous suppression of adrenal function can also depress DHEA (which can occur unexpectedly, even with steroid inhaler use). Do not cling to obsolete guidelines stating even that laboratory tests of women’s testosterone levels are not necessary and should be used only to monitor replacement therapy for deficiency – which diagnosis must be intuited, one assumes.154
How then should we diagnose low DHEA? Symptoms are generally nonspecific. Aging is not – but its consequences at a given age are individually variable. Chronic stress and altered HPA-axis can be related to fibromyalgia and persisting pain, chronic fatigue syndrome, burnout and post-traumatic stress disorder.155,156 Guidelines for diagnosing women’s hypoactive sexual desire disorder/dysfunction and female sexual arousal disorder89 and for the genitourinary syndrome of menopause are established.106
When planning tests for DHEA, bear in mind a few physiological facts: DHEA levels have strong diurnal variation, as little is bound to SHBG, while DHEA-S levels (tightly bound to the transport protein) fluctuate only a little. The ratio of these two will vary through the day. Also, large amounts of oral DHEA doses are converted to DHEA-S (a largely one-way step) in the hepatic “first pass”; consider this in monitoring therapy.
Tests are easily available for both DHEA and DHEA-S in blood, urine, and saliva. AJ Cleare found saliva the less reliable medium, commenting there is excessive variance between steroid levels in saliva and those in blood and urine.157 In blood testing at national labs, only total DHEA and DHEA-S are available; free (unbound) levels are not offered.
Knowing that excessive DHEA is conjugated to DHEA-S, it would seem plausible that the earliest marker of DHEA-deficiency may be low plasma DHEA-S. A better method may be “Metabolomics,” the study of precursors and by-products.158 This principle has been very useful in clinical practice, when applied to results of a 24-hour urine adrenal steroid profile (GC/ mass spec.). This collection removes confusion caused by the diurnal rise and fall of steroid levels.159; A similar alternative is the dried urine test for cortical hormones.
There is convenience and clinical utility in testing blood for “classical” steroid sex hormones instead of DHEA, particularly to follow the results of treatment. Remember that non-bioidentical steroid hormones (e.g., oral contraceptives, budesonide) are chemically altered and thus “invisible” to the laboratory. When test results are inexplicably low, ask about such hormone use before considering any replacement! Other pharmaceuticals can interfere; for example, ketoconazole inhibits 17-20 desmolase and reduces DHEA and its products.
Case 3 is a 42-year-old man, allergic on immunotherapy, taking “natural” thyroid for a goiter and S/P successful operation (UPPP) for sleep apnea. His residual symptoms led to an adrenal workup, which in February 1997 yielded the following: Blood tests showed elevated AM ACTH=338 H (9-52 pg/mL) and normal cortisol=16.1 (4-22 mcg/dL). Anti-adrenal autoantibody test was negative. His 24 hour-urine ACTH-stimulating test was abnormal: Baseline cortisol=28 (10-80 mg/24 Hr.), cortisone=98 (18-101 mg/24 Hr.) and androgens were low (DHEA, androsterone). After ACTH injection, his adrenal function fell: Cortisol=11 L, cortisone=34 L—what Jonathan Wright has called “the stumbling runner phenomenon.” The DHEA remained low.
He began taking DHEA 25 mg daily at bedtime and then, twice daily (morning and bedtime). His ACTH value improved to 59 H, still elevated. He started taking desiccated neonatal bovine adrenal cortex. His ACTH returned to normal, 29 and subsequently remained no higher than 37 (last follow-up 4/2021). Some years later, he briefly stopped his desiccated adrenal cortex and felt much worse. On resuming it, he promptly returned to normal.
Before Starting DHEA
Long ago, Doc found that his patients responded better to DHEA and pregnenolone when they had first started taking desiccated neonatal bovine adrenal cortex (AKA “glandular”). This hormone-free supplement is believed to provide nutrients and co-factors for the synthetic enzymes of the steroid-synthesis pathways. These enzymes are in the adrenals and ovaries; in the brain (to make neurosteroids); in the skin; and importantly, in mitochondria (to make energy). In fact, some people on starting it have bewildering energy, even a re-feeding syndrome.
For about four months in the 1990s, Doc tried giving DHEA without the adrenal glandular, hoping to save patients some money… he failed parlously. DHEA alone helped very few and not very much when it did. His patients quit taking it and refused all suggestions to start the glandular and then resume DHEA. Metaphorically, they had put gasoline in their tank without first putting oil in the engine. If the synthetic enzymes lacked necessary co-factors, the DHEA could go nowhere but out.
Informed consent is essential before treatment. Women should be cautioned that their fertility can be increased. Some who thought they were in menopause have started cycling again for a few years more. The risk of side effects should be reviewed (see the discussion following). All people with insulin resistance should know their risk of potential problems is increased, so their initial dosing will be more conservative.
Sources of DHEA
Currently available DHEA products are manufactured from yams. Years ago, the purity and potency of the commercially available products were highly irregular, with content ranging from 150% to 0% of the labeled amount.160,161 Follow-up using 24-hour urine adrenal steroid profiles show the “bad” products have elevated values of DHEA and androsterone and etiocholanolone, the products of androstenedione (which is not measured). However, testosterone was slightly lower; presumably, the “bad” precursor was blocking the pathways.
Thus, Doc used compounded DHEA when he first recommended it. When the patient had good results, a trusty health-food store owner suggested less expensive OTC supplements for comparison and thus, reliable brands were found. Currently, the quality is much improved – though questions linger around some manufacturers.
Start DHEA Supplements
Men: Once the adrenal “glandular” is at the full dose (two in the AM, perhaps one at noon and two at bedtime), patients can start DHEA. Men take 25 mg at bedtime, so it will be available in the wee hours of the morning when the HPA- and HPT-axes crank up hormone production. After a week or more, a few men need 50 mg daily. No patient has been observed to require a larger dose for a desirable outcome—or to take more than 75 mg daily without some androgenic side-effects. Some men like to divide the DHEA: Half at bedtime and half on waking; it is a matter of individual preference.
Women: Over about ten years of clinical efforts, it became apparent that most women benefit more from equally dosed pregnenolone than from DHEA. Thus, after the “glandular” is up to speed, women are offered pregnenolone 10 mg at bedtime, then 20 mg daily and sometimes more. If this does not correct low testosterone, DHEA 5 mg is recommended. On pregnenolone up to 30 or even 40 mg daily, very few women need more than 10 mg DHEA to restore total testosterone to the midst of the physiological range.
To repeat, long experience indicates the “maximum” dose of DHEA—or of DHEA combined with pregnenolone—that combines effectiveness with freedom from side-effects is about 50 mg daily. Current literature reports the common use of larger amounts: A recent meta-analysis of 42 RCTs demonstrated that testosterone level was significantly increased after DHEA administration. The gain was greater when more than 50mg/ day was administered.79 Studies cited above using DHEA to restore fertility to women with diminished ovarian reserve usually give 75 mg daily (in three divided doses). The highest dose, the most egregiously excessive dose found in the medical literature was reported in 1990, when women were given 400 mg DHEA orally four times daily – 1,600 mg a day162– wowzah!
Why does this literature show larger doses of DHEA are needed than some 27 years of clinical experience would indicate? Amidst various plausible explanations, the experience of Case 3 offers an attractive hypothesis: DHEA supplements are more beneficial at lower doses when accompanied by the nutritional cofactors necessary for the synthetic enzymes of the steroid synthetic pathways to process the DHEA.
What is the optimal dose? The optimal dose is the least that will accomplish the maximal desired response. There is a tipping point, at which undesirable side effects increase more rapidly than therapeutic benefit. We’ve seen a report in which women were given large doses of DHEA and this androgen caused “no serious side effects.”95 In fact, no report claiming such a problem was discovered. Despite the freedom from severe complications, there are reasons to dose cautiously: Excessive DHEA can cause cosmetic, behavioral, and possibly anatomical side effects.
Risks, Complications and Side-Effects
Since desiccated neonatal adrenal cortex has been recommended, it is proper to mention that patients who are significantly deficient in the nutrients it provides can have “re-feeding syndrome” on starting it. This has been discussed in detail elsewhere.163 Similarly, as Paracelsus wrote: “The dose makes the poison”164and complications of DHEA are related to the dose given and the rate of its increase.
In a two-year-long randomized, prospective, placebo-controlled double-blinded study of DHEA supplementation, older men took 75 mg daily and women took 50 mg daily. Prostate volume, PSA, liver function, electrolyte levels and hemoglobin were not significantly worse in the treated group compared to controls.74 Meta-analysis of the evidence supporting the use of systemic DHEA in postmenopausal women with normal adrenal function reported no serious adverse effects and no worsened serum lipids, serum glucose, weight, or body mass index.165 Intensivists caring for severe trauma victims report: “The current data suggest that DHEA, certainly in short-term supplementation, should be regarded as safe without significant side effects.”166
In practice, the most common clinical side effects of DHEA treatment are caused by the fact that it is effective. Doc calls them “the three A’s of androgens”: First is attitude, due to the restoration of testosterone and neurosteroids. A positive, outgoing and assertive outlook are desirable, but some men become aggressive and irritable—downright disagreeable. When this occurs, they are getting too much testosterone too quickly. Reduce the dose and build it back more slowly. Perhaps the androgen receptor population is “over-expressed” when testosterone is deficient, as is the thyroid receptor in hypothyroidism.167-169 An unusually large number of “hungry” nuclear receptors can excessively respond to the rapid replenishment of a normal amount of hormone.
The second “A” is alopecia. This also can be physiological, the predictable result when normal testosterone is converted normally to DHT in men with the gene for male-pattern hair loss. Unfortunately, women with PCOS and insulin resistance over-express 5alpha-reductase and can excessively produce DHT, sometimes with the same effect.10 Doc found that women with PCOS get androgenic side effects on as little as 15 mg DHEA daily, even when taking the “glandular.”
The third “A” is acne, another consequence of DHT in the skin. A meta-analysis of 16 RCTs in which women were given DHEA for perimenopausal or menopausal symptoms reported increased androgenic side effects (odds ratio 3.77), principally acne. This occurred whether DHEA was taken orally or applied topically.170 Experience indicates that women with PCOS and insulin resistance, who already tend to hyperandrogenemia, seem most vulnerable.
Hirsutism is also an undesirable androgenic complication. Watch for darkening hair on the upper lip and sides of the chin; also circumareolar hair and down the hypogastric midline. These hairs do not always regress after DHEA is withdrawn. If this seems trivial to the practitioner, few affected patients share that perspective.
As previously noted, the restoration of adrenal nutrition and sex hormone precursors can increase fertility and restore normal menstrual cycling. This is not considered a side-effect unless an unwanted pregnancy occurs. Some patients have not been happy about the resumption of their menses.
Though DHEA has relatively low affinity for the androgen receptor, it and its androgenic byproducts can compete with DHT and testosterone for receptor binding sites. Conceptually, the more DHEA in circulation, the less the androgen receptor may be able to act, due to binding the low-potency precursors.
Laboratory follow-up also shows that older men particularly will convert excess DHEA via androstenedione to estrone. This estrogen can cause prostate enlargement by stimulating medullary estrogen receptors,171 particularly if it is configured as 16α hydroxy-estrone.172 This also could affect the incidence of malignancy.173
Metabolic syndrome patients are endocrine disrupted, which can lead to apparent treatment failure. Women’s elevated 5a-reductase excessively produces DHT, as above.10 Men have increased aromatase174 that doubles the risk of low-testosterone in metabolic syndrome,175while almost 50% of men with type-2 diabetes have low testosterone.176 Supplemental DHEA will increase their androgen production …but these promptly will be converted to estrogens. Limit the amount of DHEA you pour in: There is “a hole in the bottom of their bucket.”
Monitor Treatment Results
The route of administration is relevant. A study of intravaginal DHEA suggested that women using vaginal DHEA “should not have blood hormone levels checked, as the serum concentration of sex steroids is minimally affected by this route of administration.”76
When monitoring is indicated, it is proper to be consistent and use the same body fluid for following therapeutic outcomes that was used to diagnose the problem. Blood tests are useful, convenient, and covered by insurance in Doc’s practice. If saliva was your initial medium, then use it for follow-up.
If you began with a 24-hour urine adrenal steroid profile, follow-up with another. Using metabolomics, excess supplementation is first revealed in the 24-hour urine by high amounts of its products: DHEA-S and importantly, androsterone and etiocholanolone (from androstenedione). On larger doses, we can see high DHEA.
When you have chosen your pre-treatment tests wisely, they need only to be repeated to validate outcomes. Because oral DHEA is so greatly converted to DHEA-S in the liver first-pass, it is sensible to follow therapeutic levels with unconjugated DHEA. Since DHEA augments testosterone, measure it too! Because testosterone is the precursor of estradiol and DHT, they may be tested – at least once.
Knowing that age increases SHBG (sex hormone-binding globulin) and metabolic syndrome reduces it, Doc orders blood tests for both total testosterone (by LC/MS) and free testosterone (by ED) – at least for the first follow-up. Age, obesity and metabolic syndrome increase men’s aromatase, so it is wise also to test total estradiol (by LC/MS). Sometimes it is indicated to test women’s DHT also, for comparison to total testosterone.10
Guidelines for monitoring testosterone therapy in women might be relevant for DHEA. The Endocrine Society suggested testing after three to six weeks on treatment to avoid toxicity and excessive dosing.90 It is reassuring that the “Global consensus” stated: “When serum testosterone levels remain in normal physiologic ranges, studies show that neither oral nor nonoral testosterone (implied “androgen”) therapy significantly affects the lipid profile, glycemic markers, blood pressure, body mass index, or hematocrit.”76
Alternatives to DHEA
7-keto DHEA is chemically-modified DHEA (Figure 3). This alteration prevents it from becoming converted to testosterone and estradiol. Most users entering Doc’s practice have been women, who say they use it to gain the benefits of DHEA without making testosterone. They hope, as enumerated by RxList, to speed up the metabolism and heat production to promote weight loss; to improve lean body mass and build muscle; to increase the activity of the thyroid gland, boost the immune system, enhance memory, and slow aging.177 Athletes have used it, believing it was an undetectable androgenic “doping” agent but current testing technology can now identify the substance178 and it has been banned by some organized sports.
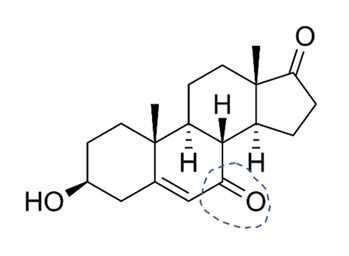
Figure 3. 7-Keto DHEA
https://en.wikipedia.org/wiki/7-Keto-DHEA#/media/File:7-Keto-DHEA.svg
The 24-hour-urine adrenal steroid profile of people taking 7-keto DHEA resembles that of “bad” DHEA (as above). As an advocate of using biologically identical hormones and precursors, Doc is uncomfortable with this peculiar strategy. He has yet to see anyone who clearly benefitted from taking it—and acknowledges that he would not see people for whom it relieved all their problems.
Pregnenolone has been mentioned above as the universal precursor of adrenal-gonadal steroids.179 In research and clinical use, its supplementation can increase progesterone production,7,180-182 along with its benefits of replenishing steroid pathways,183 reduction of pain and the stress response184 and a mild GABAergic effect.185 Feedback inhibition from downstream products (DHEA, androstenedione, estrone and testosterone) inhibits the enzyme (3β-HSD) that converts pregnenolone to progesterone.7,70,96 Thus, it is remarkably free of any effects besides those caused by replenishment of cortisol, aldosterone, progesterone and the other sex hormones (which can be increased uncomfortably quickly).
As above, women respond to pregnenolone supplementation well, unless excessive 5alpha-reductase converts it excessively to the neurosteroid, allopregnanolone, which in menopausal women, can cause headaches.10 Doc had a small series of middle-aged men who tried 10 mg pregnenolone at bedtime. Everyone liked it (perhaps from the GABA effect) but most men’s blood levels of progesterone became too high, up to 3 ng/dL (0.3-1.2). This caused mild gynecomastia and perhaps increased abdominal adiposity. It is likely that 5 mg daily would be successful.
4-Androstenediol (Figures 1 and 4), has been used to supplement testosterone production. In fact, its use for this purpose was patented by Patrick Arnold in 1998, then allowed to expire in 2018. Doc has no experience of using this precursor. Its naturally occurring near-twin nandrolone is normally present in the human body fluids in trace amounts. Nandrolone also can be found some physicians’ offices with other parenteral medications, but it has no role in the author’s practice.
Figure 4.
Testosterone and estradiol are the ultimate products of DHEA. It was an early disappointment to Doc that, while he could achieve follicular-phase levels of progesterone with OTC pregnenolone and normal women’s testosterone values with DHEA, neither one could restore follicular blood levels of estradiol. Some patients, particularly those with primary gonadal failure need to receive these hormones.
Summary
Dehydroepiandrosterone (DHEA) is neither a miracle hormone nor a wonder drug. It is a moving part in a vehicle that is wonderfully constructed. It serves as a precursor and an agonist of both genomic and non-genomic messages. Its deficiency is noteworthy.
In ordinary circumstances, insufficient DHEA is troublesome. With age, its decline is associated with many degenerative changes. When the maladaptive stress responses of illness, pain, or injury have inhibited DHEA production, significantly ill consequences can follow.
With some understanding—and having prepared the patient with desiccated neonatal adrenal cortex to receive DHEA as a farmer fertilizes his fields—correctly dosed DHEA supplementation can be very beneficial.
Author bio:
Alan McDaniel, MD is a 1977 Tulane medical graduate. He trained in General Surgery and Emergency Medicine before becoming Board-certified in Otolaryngology with sub-specialties in Neurotology and Allergy. He has practiced privately since a two-year faculty appointment at the University of Louisville.
He has presented at various national meetings in the U.S. (AAO-HNS, AAOA, ANS, AAEM, IFM, PAAS, ACAM, ICIM) and in Mexico. Topics of his lectures and publications have included General Surgery and Otolaryngology; Otology and Neurotology; Allergy; Chronic fatigue and Endocrinology. He has been a faculty member for the American Academy of Otolaryngic Allergy Basic and Advanced Courses and for the American Academy of Environmental Medicine. His two-day course “New Endocrinology” has been presented at the AAEM and elsewhere since 2005, to physicians from five continents.
Work with dizziness and allergy in the 1980s led him to seek solutions for Chronic Fatigue Syndrome. These investigations were extended to the endocrine aspects of this and related conditions. Since basic surgical training emphasizes the need to know several alternative approaches to an operation, he saw the logic of studying integrative and controversial medical methods. He has endeavored to understand these in the light of new facts from research, mindful that Medical history shows innovation begins as a minority opinion.
He is excited that applying new research to patient care offers solutions to many of the chronic and worsening problems that are epidemic in modern society.
Consult your doctor before using any of the treatments mentioned in this article.
Reprinted with permission from the February/March 2022 Townsend Letter and Alan B. McDaniel, MD.
Learn how you can benefit from more Townsend Letter articles.